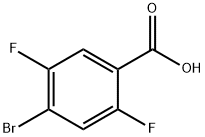
4-bromo-2,5-difluorobenzoic acid: Preparation and Applications in Bioorganic Chemistry
General Description
4-Bromo-2,5-difluorobenzoic acid is a synthetic chemical compound that is widely used in scientific research. It is a versatile compound that can be used in many different laboratory experiments. It is a fluorinated benzoic acid derivative2 and can be used as a reagent in a variety of organic and inorganic reactions. It is also a relatively inexpensive compound, which makes it an attractive option for laboratory experiments. However, it is important to note that 4-Bromo-2,5-difluorobenzoic acid is a fluorinated benzoic acid derivative and can act as a catalyst in a variety of reactions. This means that it can react with other compounds in the reaction mixture, which can lead to undesired side reactions.

Figure 1. Properties of 4-Bromo-2,5-difluorobenzoic acid
Preparation
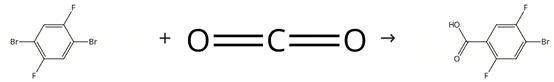
Figure 2. Synthesis of 4-Bromo-2,5-difluorobenzoic acid
Freshly titrated n-butyl lithium (27.0 ml, 1.39 M in hexanes) is added slowly (over about 30 minutes) to a -78°C solution of diethyl ether (90 ml) containing 1,4-dibromo-2,5-difluorobenzene (10.22 g, 0.038 mol). The resulting yellow solution is stirred at -78°C for 2 hours to give a yellow suspension. Several pellets (~10) of dry ice are added to the suspension, which is then allowed to warm slowly to room temperature as it degasses (approximately 40 minutes). The resulting suspension is acidified with a 1 M aqueous solution of hydrochloric acid (500 ml), and the product extracted with diethyl ether (5 x 200 ml). The combined organics are washed with water (4 x 100 ml) and filtered. The ether solution is concentrated to approximately 200 ml under reduced pressure, and the product extracted into a saturated aqueous solution of sodium bicarbonate (3 x 200 ml). The combined aqueous extracts are washed with methylene chloride (3 x 100 ml) and acidified with hydrochloric acid. The product is extracted with diethyl ether (3 x 200 ml), and the combined organic extracts washed with water (2 x 200 ml), dried over magnesium sulfate, and concentrated under reduced pressure to give 4-Bromo-2,5-difluorobenzoic acid as a pale yellow solid.1
Applications
Intermediate in Preparation of GPR119 Agonists
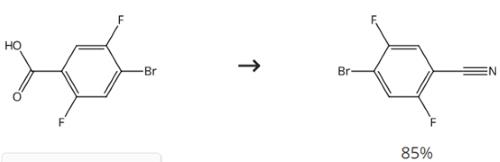
Figure 3. Intermediate in Preparation of GPR119 Agonists
Stir a mixture of 4-bromo-2,5-difluorobenzoic acid (53.28 g), thionyl chloride (165 mL) and DMF (0.87 mL) at 80 °C for 1.5 h. Cool down the mixture to room temperature. Evaporate the mixture in vacuo. Dissolve the resulting residue in chloroform (300 mL). Add 28 wt % aqueous ammonia (300 mL) dropwise to the solution at 5 °C. Stir the mixture at 5 °C for 0.5 h. Extract the mixture with chloroform. Dry the organic layer. Remove the desiccant by filtration. Evaporate the filtrate in vacuo to obtain a pale yellow solid. Stir the mixture of the obtained solid and phosphoryl chloride (195 mL) at 80 °C for 2 h. Cool down the mixture to room temperature. Evaporate the mixture in vacuo. Treat the resulting residue with diethyl ether (500 mL) and ice-water (300 mL). Stir the residue at room temperature for 0.5 h. Extract the mixture with diethyl ether. Wash the organic layer with saturated aqueous sodium hydrogen carbonate and brine. Dry the organic layer. Remove the desiccant by filtration. Evaporate the filtrate in vacuo to obtain 4-bromo-2,5-difluorobenzonitrile.2
Intermediate in Preparation of GPR119 Agonists
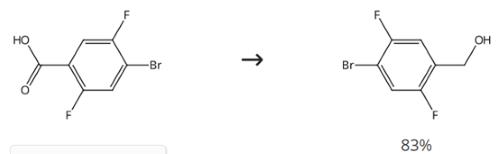
Figure 4. Intermediate in Preparation of Selective METTL3 Inhibitors
Add BH3·Sme2 (2 equivalents, 8.4 mmol, 4.2 mL, 2 M THF) to a stirred solution of 4-bromo-2,5-difluorobenzoic acid (1 g) in dry THF (10 mL) under a nitrogen atmosphere. Stir the reaction mixture for 17 hours at 25°C. Cool down the reaction mixture to 0°C. Quench the reaction mixture by the addition of a saturated aqueous Na2CO3 solution. Extract the aqueous layer three times with EtOAc. Wash the combined organic layers once with brine. Dry the combined organic layers over MgSO4. Concentrate the combined organic layers under reduced pressure.3
Intermediate in Preparation of Selective NaV1.7 Inhibitors
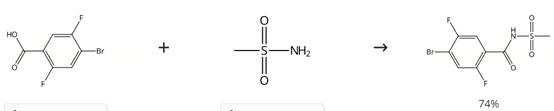
Figure 5. Intermediate in Preparation of Selective NaV1.7 Inhibitors
Charge a round-bottomed flask with 4-bromo-2,5-difluorobenzoic acid (10.00 g), methanesulfonamide (4.82 g, 50.6 mmol), DMAP (11.34 g, 93 mmol) and EDC·HCl (16.18 g, 84 mmol). Add CH2Cl2 (150 mL) to the mixture. Stir the reaction mixture for 2 day at room temperature. Concentrate the mixture under reduced pressure. Dissolve the crude material in 500 mL of CH2Cl2 and 10 mL of MeOH. Filter over 25 μm filter paper. Inject the crude solution onto a 1500 g silica gel cartridge (40-63 μm). Elute at 300 mL/min with a gradient over 10 column volumes (15 L total volume) from 2/98/0.1 MeOH/CH2Cl2/formic acid to 20/80/1 MeOH/CH2Cl2/formic acid.4
References
1. WO2012106995 A1 2012-08-16.
2. Negoro, Kenji; et al. Bioorganic & Medicinal Chemistry (2012), 20(17), 5235-5246.
3. Dolbois, Aymeric; et al. Journal of Medicinal Chemistry (2021), 64(17), 12738-12760.
4. DiMauro, Erin F.; et al. Journal of Medicinal Chemistry (2016), 59(17), 7818-783.
Related articles And Qustion
Lastest Price from 4-bromo-2,5-difluorobenzoic acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 28314-82-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt