Propyl Gallate: Overview, Physiochemical Properties and Analysis Methods
General Description
Propyl gallate is a widely used antioxidant with applications in food, cosmetics, and pharmaceuticals, known for preventing lipid peroxidation and enhancing product stability. Regarded as safe by authorities like the FDA, its safety continues to be assessed by the European Commission due to potential toxicity under specific conditions. With a chemical formula of C10H12O5, propyl gallate has unique physiochemical properties that aid its incorporation into various formulations. Traditional analysis methods, such as chromatography, are challenged by complex antioxidant profiles in foods. Emerging techniques like electrochemical sensors and fluorescence probes offer more efficient and sensitive alternatives for propyl gallate detection.

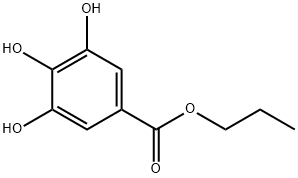
Figure 1. Propyl gallate
Overview
Introduction of Propyl Gallate
Propyl gallate is a widely utilized antioxidant derived from gallates, specifically known for its role in controlling lipid peroxidation. This compound is frequently employed in the food industry, cosmetics, and pharmaceuticals due to its ability to maintain the freshness, color, and aroma of products. Propyl gallate has been in use for over 70 years and is recognized for its efficacy in preventing the degradation of fats and oils. Beyond its primary function as an antioxidant, recent studies have highlighted its antimicrobial properties, adding to its versatility in various applications. 1
Safety and Regulatory Aspects
Propyl gallate is generally considered safe when used within authorized limits, as per the standards set by the U.S. Food and Drug Administration and Good Manufacturing Practices. Despite its long history of use, the European Commission has called for ongoing assessment of its safety, pharmacokinetics, and toxicological properties. While several animal studies have not found carcinogenic effects of Propyl gallate, some research has indicated potential toxicity under certain conditions. Additionally, Propyl gallate has been investigated for therapeutic benefits, including its chemotherapeutic, nephroprotective, and cytotoxic effects. 1
Physiochemical Properties
Propyl gallate, a derivative of gallic acid, plays a significant role as an antioxidant in various applications. The core structure of propyl gallate, derived from gallic acid, features three hydroxyl groups that contribute to its effectiveness as a polyphenolic compound. This additive, characterized by the chemical formula C10H12O5, exhibits unique physiochemical properties that enhance its usability in food preservation and safety. The molecular weight of propyl gallate is 212.20 g/mol, with a density of approximately 1.36 g/cm³. The compound displays a melting range of 146–148 °C and a boiling point of around 181 °C. Additionally, propyl gallate has a pKa of 7.94 and a pH of 6.3 when in a 0.05% aqueous solution, indicating its stability in various pH environments.
In terms of solubility, propyl gallate exhibits low water solubility, dissolving at only 1 mg/mL at 20 °C. However, it is more readily soluble in organic solvents such as ethanol, ether, and propane-1,2-diol, which allows for its effective incorporation into lipophilic formulations. The hydrophobic nature of propyl gallate, a result of its acylation, contrasts starkly with its parent compound, gallic acid, enhancing its application in fats and oils. As an approved food additive in the European Union and several other countries, propyl gallate is recognized by the E-number E310. Its utility goes beyond food preservation, where it minimizes oxidation and enhances shelf life, making propyl gallate a valuable ingredient in various cosmetic and pharmaceutical products as well. 2
Analysis Methods
Analytical Techniques for Propyl Gallate Detection
Analyzing propyl gallate in food products is crucial for ensuring compliance with food safety regulations. Several analytical methods are employed to measure the concentration of propyl gallate, including high-performance liquid chromatography, gas-liquid chromatography, and ultraviolet-visible spectrophotometry. These methods require specialized equipment and trained personnel, making them relatively costly and time-consuming. Recently, innovative techniques such as thin-layer chromatography and capillary electrophoresis have also been utilized. However, these approaches often involve challenges due to the presence of multiple phenolic antioxidants in food samples, complicating the specific detection of propyl gallate. The complexity and overlap of antioxidant profiles necessitate highly sensitive and precise methods for accurate analysis. 2
Emerging Methods
Given the challenges associated with traditional detection methods for propyl gallate, researchers have begun exploring alternative techniques. Electrochemical sensors, fluorescence probes, and colorimetric devices are emerging as viable options due to their affordability, rapid analysis times, and high sensitivity. Among these, electrochemical methods have garnered increased attention for their ability to analyze various samples effectively, including opaque materials. For instance, recent studies have demonstrated dual-radiometric electrochemical sensors achieving low limits of detection for propyl gallate, highlighting their potential for application in real-world food testing scenarios. Innovations like using nickel nanoparticles and modified electrodes not only enhance detection sensitivity but also improve the overall efficiency of propyl gallate analysis in various food matrices, paving the way for more practical monitoring of this important food additive. 2
References:
[1] FATEMEH JAVAHERI-GHEZELDIZAJ . Pharmacokinetic and toxicological overview of propyl gallate food additive[J]. Food Chemistry, 2023, 423. DOI:10.1016/j.foodchem.2022.135219.See also
Lastest Price from Propyl gallate manufacturers

US $1.00-4.00/KG2025-09-02
- CAS:
- 121-79-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $100.00-12500.00/kg2025-06-06
- CAS:
- 121-79-9
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg