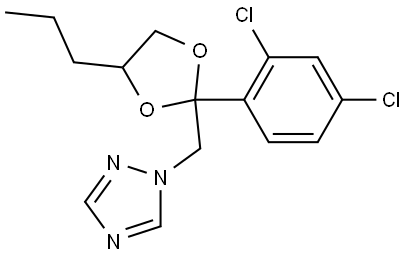
Propiconazole: mammalian toxicity, ecotoxicology and residues
General Description
Propiconazole, an active substance used in pesticides, has been extensively evaluated for mammalian toxicity. It exhibits low acute toxicity when applied dermally or inhaled, and moderate toxicity when administered orally to rats. The liver is identified as the target organ in rodents, while the pituitary gland may be affected in dogs. Propiconazole does not cause mutagenicity or carcinogenicity in humans. However, it may lead to skin sensitization and reproductive toxicity. Further investigation is required regarding its potential endocrine-disrupting properties. Ecotoxicological assessments indicate low risks to honeybees, wild mammals, aquatic organisms, non-target arthropods, soil organisms, and terrestrial plants. Its residue assessment reveals the need for defining residue definitions for different crops and addressing chiral analysis and isomeric degradation information.

Figure 1. Propiconazole
Mammalian toxicity
Propiconazole is an active substance used in pesticides, and its mammalian toxicity has been extensively evaluated. It is rapidly absorbed and primarily eliminated through bile excretion. The main metabolic pathway involves oxidation, cleavage, and hydroxylation reactions. Propiconazole exhibits low acute toxicity when applied dermally or inhaled, and moderate toxicity when administered orally to rats. It does not cause skin or eye irritation but may lead to skin sensitization. Short-term oral toxicity studies have identified the liver as the target organ in rodents, while the pituitary gland may be affected in dogs. Genotoxicity studies show no mutagenicity but uncertainties remain regarding clastogenic and aneugenic potential. Long-term studies reveal liver and adrenal toxicity in mice and rats, respectively. However, liver tumors observed in male mice are not a concern for humans, and propiconazole is not considered carcinogenic. Reproductive toxicity studies indicate decreased litter size and viable pup numbers at higher doses. Propiconazole is classified as toxic for reproduction category 1B and may possess endocrine-disrupting properties, requiring further investigation. The acceptable daily intake is set at 0.04 mg/kg body weight per day, with an acute reference dose of 0.1 mg/kg body weight. Exposure assessments show levels below the acceptable operator exposure level without personal protective equipment. Further investigations are recommended on isomeric composition, metabolites, and groundwater impacts. In summary, propiconazole demonstrates various toxicological effects, including target organ toxicity, reproductive toxicity, and potential endocrine disruption. It is important to address data gaps and uncertainties through further studies and assessments. 1
Ecotoxicology
Propiconazole is a pesticide that has undergone risk assessment based on various documents including those by the European Commission, SETAC, and EFSA. The risk assessment considered the acute and chronic toxicity to honeybees, as well as the development of honeybee brood and larvae. However, a risk assessment scheme for chronic toxicity data in adult honeybees and bee brood was lacking, so the EFSA (2013c) was used instead to reach a conclusion for representative uses. For wild mammals, a low acute and long-term risk through dietary exposure and secondary poisoning was concluded based on available data and risk assessment. In terms of aquatic organisms, a low acute and chronic risk was determined for all uses of propiconazole. However, the presence of four stereoisomers in propiconazole raised uncertainty regarding specific toxicity and exposure. To account for this uncertainty, a further factor of 4 was suggested. The assessment of metabolites indicated low acute and chronic risks, but uncertainty remained for metabolites with isomers. A data gap was identified in this regard. Regarding non-target arthropods, a low risk was concluded based on tier I and tier II studies, with no significant treatment-related effects observed. For soil organisms, non-target terrestrial plants, and biological methods of sewage treatment, a low risk was concluded based on available data. Regarding endocrine disruption potential, evidence suggested that propiconazole acts as an aromatase inhibitor in fish, leading to reproductive dysfunction. However, further assessment is required to determine its potential endocrine disruptive properties. 2
Residues
The assessment of propiconazole residues is based on various guidance documents and recommendations, including those from the OECD, European Commission, and Joint Meeting on Pesticide Residues (JMPR). Residue studies have been conducted on various crops, revealing propiconazole as the main component of total radioactive residues in grapes, rice, carrots, and celery. Different metabolites, such as CGA118244 and CGA91304, were identified in various crops, leading to discussions on the residue definition for monitoring and risk assessment. Rotational crop metabolism studies indicated a different metabolic pathway compared to primary crops, creating a data gap for defining residue definitions for rotational crops. In livestock, CGA91305 was identified as a valid residue marker, and further feeding studies are needed to address the full livestock residue definition. The current consumer risk assessment only considers propiconazole and plant matrices, pending the completion of toxicological assessments and the inclusion of relevant compounds in the residue definition for risk assessment. Additionally, chiral analysis and isomeric degradation information are lacking, adding uncertainty to the consumer exposure assessment. 1
Reference
1. European Food Safety Authority (EFSA), Arena M, Auteri D, et al. Peer review of the pesticide risk assessment of the active substance propiconazole. EFSA J. 2017;15(7):e04887.
2. European Food Safety Authority (EFSA), Adriaanse P, Arce A, et al. Revised guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2023;21(5):e07989.
You may like
Related articles And Qustion
Lastest Price from Propiconazole manufacturers

US $0.00/kg2025-09-28
- CAS:
- 60207-90-1
- Min. Order:
- 1kg
- Purity:
- 95
- Supply Ability:
- 200000

US $0.00/KG2025-04-21
- CAS:
- 60207-90-1
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month