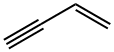
Process for manufacture of vinylacetylene
Process for manufacture of vinylacetylene
Acetylene is dimerized in the presence of hydrochloric acid solution of cuprous chloride and ammonium chloride to obtain vinylacetylene.
Acetylene dimerization
Acetylene dimerization reaction is usually carried out in a bubbling bed reactor. Acetylene gas is continuously introduced into a liquid solution containing the catalyst, acetylene gas undergoes a dimerization reaction to yield vinylacetylene. The unreacted acetylene gas and the generated vinylacetylene flow out of the reactor together, and are separated in a subsequent separation system to obtain a product containing small amounts of impurities. The unreacted acetylene is returned to the feed system. The reaction equations are as follows:
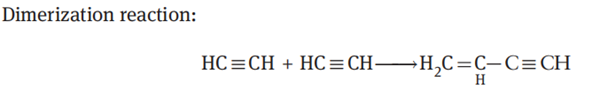
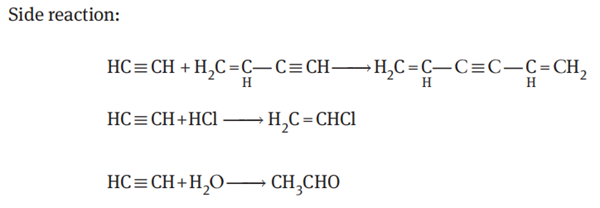
Among them, vinylacetylene is the main product, and there are some by-products including a small amount of divinylacetylene, vinyl chloride, acetaldehyde and a trace of oligomers with high boiling point.
The Nieuwland catalyst has been widely used in the dimerization of acetylene
due to its simple preparation and application, mild reaction conditions, good safety
and low cost.
Acetylene dimerization catalyst
1. Nieuwland catalyst
The Nieuwland catalyst is prepared by dissolving the active component cuprous chloride together with a co-solvent in deionized water. It is suggested that the copper chloride complex ions (CuCl2−, Cu2Cl3−, Cu3Cl4−, Cu4Cl5−) play a catalytic role in the reaction.
The co-solvent of the catalyst is a chlorine-containing ionic compound. Chlorine ion and cuprous chloride form a complex ion to promote its dissolution. The type and dosage of co-solvent are important factors affecting the catalytic effect. Usually, KCl and NH4Cl are used as the co-solvent. The free Cl− in the catalyst solution inhibits the dimerization of acetylene. Therefore, in order to maintain sufficient activity of the Nieuwland catalyst, the amount of the co-solvent should not be too much. The optimal dosage of the co-solvent is just enough to make CuCl complete dissolution in water.
The dimerization of acetylene in the presence of the Nieuwland catalyst requires very strict acidity, and its adjustable range is narrow. The dimerization of acetylene is retarded by hydrogen ion in the catalyst solution, and the higher the concentration of hydrogen ion, the lower the dimerization rate of acetylene. The mass fraction of hydrogen ion in the catalyst system should not exceed 0.1%, otherwise the by-products will increase, and the conversion of acetylene and the selectivity to vinylacetylene will decrease.
The preparation of the Nieuwland catalyst includes a two-step process and a one-step process. In two-step process, copper reacted with chlorine in hydrochloric acid to prepare a wet cuprous chloride with a water content of 23–30%; then, the resultant wet cuprous chloride was mixed with ammonium chloride and other components, and the mixture was heated under stirring to obtain the catalyst solution. The two-step process for preparation of the Nieuwland catalyst was mature, but it took a long time, and the utilization of copper in the preparation of wet cuprous chloride was only 76–81%. The preparation had been improved by one-step process, in which electrolytic copper blocks and hydrochloric acid was added to aqueous ammonium chloride solution, and chlorine was introduced into the solution under stirring. The activity of the catalyst prepared by one-step process was almost the same as that by two-step process, but the preparation time was shortened and the copper was fully utilized in the one-step process.
2. Nonaqueous phase catalyst for acetylene dimerization
At present, the industrial production of vinylacetylene mainly uses aqueous reaction system, but the conversion of acetylene per pass is only about 10%, and various
by-products are also generated. Dimerization of acetylene in nonaqueous phase
reaction system was proposed by DuPont Company. In this system, the conversion of
acetylene is higher, and no hydration reactions of acetylene or vinylacetylene occur,
resulting in less by-products.
In the nonaqueous phase catalyst, cuprous chloride is still used as the active component, a primary or secondary aliphatic amine hydrochloride is used as a co-solvent.
Cuprous chloride and the co-solvent are dissolved in an organic solvent having
excellent solubility for acetylene. Usually, the preferred co-solvents are ethylamine
hydrochloride and dimethylamine hydrochloride, while the best solvent is dimethylformamide. For example, in the presence of a catalyst system prepared by dissolving 140 g of cuprous chloride and 70 g of dimethylamine hydrochloride in 150 mL of
dimethylformamide, acetylene dimerization reaction was carried out at a temperature
of 65°C and acetylene space velocity of 100 h−1, resulting in acetylene conversion of
20% and the selectivity to vinylacetylene of 92%. In another research, the optimum
catalyst solution containing 53 g of CuCl, 25 g of dimethylamine hydrochloride and
54 mL of dimethylformamide was used, and the reaction was performed at a temperature of 67°C and acetylene space velocity of 320 h−1. The conversion of acetylene and
the selectivity to vinylacetylene reached 15.53% and 94.6%, respectively
3. Acetylene dimerization reaction condition
The effects of reaction conditions on the dimerization process of acetylene are mainly reflected in three aspects: reaction temperature, acetylene space velocity and acetylene pressure. In the dimerization of acetylene, the increase of temperature will aggravate the occurrence of side reactions, resulting in the decrease of selectivity of vinylacetylene; However, if the temperature is too low, the reaction rate is very slow, so that although high selectivity can be obtained, the production capacity is too small, which is also not conducive to actual production. Jiao et al. showed that 80°C was the optimal reaction temperature in aqueous system by orthogonal experiment; In the nonaqueous system with dimethylamine hydrochloride as the promoter, yellow needle-like crystals were formed when the temperature was lower than 65°C, and insoluble substances were precipitated when the temperature was higher than 70°C due to the increased volatilization of the solvent, so the reaction temperature was selected as 67°C.