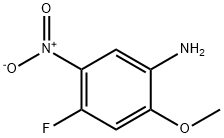
Preparation of 4-fluoro-2-Methoxy-5-Nitroaniline
4-fluoro-2-Methoxy-5-nitroaniline(4-fluoro-2-methoxy-5-nitro-phenylamine, 4-fluoro-2-methoxy-5-nitrophenylamine, 2-methoxyl-4-fluoro-5-nitroaniline) is an important organic intermediate to synthetize substituted benzene products.4-fluoro-2-Methoxy-5-nitroaniline can be prepared according to the reported literatures[1-6].
Method 1
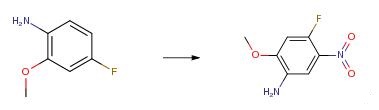
The starting material (551 mg, 3.90 mmol) is dissolved in dichloromethane (39.0 mL), concentrated sulfuric acid (1.85 mL) is added dropwise with stirring under ice-cooling, and then concentrated nitric acid (267 μL, 5.85 mmol) is added dropwise. After stirring for 3 hours under ice-cooling, saturated aqueous sodium bicarbonate was added until pH 8 was reached. After washing with saturated aqueous sodium bicarbonate solution, washing with saturated brine, the organic layer was dried over sodium sulfate and evaporated under reduced pressure to obtain 675 mg of the product (yield 93%).
Method 2
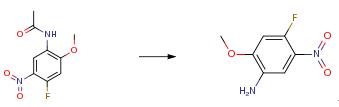
In a clean and dry round bottom flask methanol (400 ml) and N-(4-fluoro-2- methoxy-5-nitrophenyl)acetamide (400 g) were added. Hydrochloric acid was added to the reaction mass at 25-35°C. The reaction mass was then heated to reflux and stirred at reflux for 3.0-5.0 hrs. The solvent was completely distilled out under vacuum, and cooled the reaction mass to 10°C and stirred for 2.0-3.0 hrs. The solid was filtered and taken into another round bottom flask then water (500 ml) added. The reaction mass pH was adjusted to 9.0 with NaOH solution and the reaction mass was extracted with ethyl acetate (2000 ml). The organic layer was washed with brine solution (NaCl (100 g) + water-2(500 ml)) and dried over sodium sulphate. The solvent was distilled out under vacuum. Petroleum ether (1000 ml) was added to the residue, cooled to below 10°C and stirred for 30 minutes. The solid was filtered, and washed with petroleum ether (250 ml) and finally dried at 50-60°C for 3-5 hrs (Yield - 240 g; 73.55 percent).
Method 3
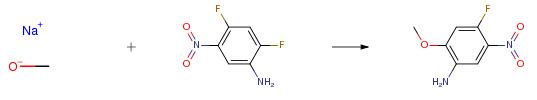
Dissolve 2,4-difluoro-5-nitroaniline (3.48 g, 20 mmol) in anhydrous methanol (50 mL). Sodium methoxide (1.30 g, 24 mmol) is added at room temperature. And stir for 48 hours. After the reaction is over, add water (100 mL), extract with dichloromethane (100 mL x 3). Combine the organic phase, The organic phase was washed with saturated brine (100 mL). Drying with anhydrous sodium sulfate, concentrate under reduced pressure, the crude product was isolated and purified by column chromatography on silica gel (eluent: petroleum ether: ethyl acetate = 6:1). A red solid (3.26 g, yield: 87.6percent) was obtained.
References
1. Kanazawa University; Ogawa K, Sobuku T, Mishiro K, Odani A. The therapeutic effect of the anticancer agent for predicting alternative (by machine translation). JP2019/43882[P], 2019, A,Paragraph 0059-0063.
2. AUROBINDO PHARMA LIMITED, CHANDIRAN T, MEENAKSHISUNDERAM S, SREENIVASA REDDY M. A Process for the Preparation of 4-Fluoro-2-Methoxy-5-Nitroaniline. WO2018/207120[P], 2018, A1,Page column 13.
3. Tianjin Binjiang Pharmaceutical Research And Development Co., Ltd. Tian H, Huang G, Cheng Y. Pyrimidine heterocyclic compound and its preparation method and application (by machine translation), CN107793413[P], 2018, A, Paragraph 0168-0169, 0172-0173.
You may like
Related articles And Qustion
See also
Lastest Price from 4-fluoro-2-Methoxy-5-nitroaniline manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1075705-01-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00/kg2025-04-21
- CAS:
- 1075705-01-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton