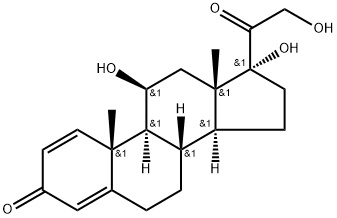
Understanding the Mechanism, Pharmacokinetics, and Side Effects of Prednisolone
General Description
Prednisolone, a corticosteroid, exerts its mechanism of action by binding to the glucocorticoid receptor. This inhibits pro-inflammatory signals and promotes anti-inflammatory signals. It reduces vasodilation, capillary permeability, and leukocyte migration. Corticosteroids also affect gene expression, inhibiting neutrophil apoptosis, arachidonic acid formation, and inflammatory transcription factors, while promoting anti-inflammatory gene expression. Higher doses have immunosuppressive effects. Prednisolone is orally administered with a bioavailability of 70%. It reaches peak concentration within 1.0-2.6 hours and has a protein binding range of 65-91%. Metabolism involves conversion to prednisone and various metabolites, excreted primarily in urine. Side effects of prednisolone overdose include gastrointestinal disturbances, insomnia, and restlessness. Management includes reducing dose or adjusting treatment schedule. LD50 values in animal models differ from human TDLO values. Ophthalmic administration is not expected to cause problems.
Figure 1. Tablets of prednisolone
Mechanism of action
Prednisolone, a corticosteroid, exerts its mechanism of action by binding to the glucocorticoid receptor. This binding inhibits pro-inflammatory signals and promotes anti-inflammatory signals. In the short term, corticosteroids decrease vasodilation and permeability of capillaries, as well as inhibit leukocyte migration to sites of inflammation. Binding to the glucocorticoid receptor leads to changes in gene expression, resulting in various downstream effects over hours to days. For example, corticosteroids inhibit neutrophil apoptosis and demargination, decrease the formation of arachidonic acid derivatives by inhibiting phospholipase A2, and inhibit inflammatory transcription factors like NF-Kappa B. Additionally, they promote the expression of anti-inflammatory genes such as interleukin-10. The anti-inflammatory effect of corticosteroids is observed at lower doses, while higher doses have an immunosuppressive effect. Prolonged use of high doses can also lead to binding with the mineralocorticoid receptor, causing an increase in sodium levels and a decrease in potassium levels. Patients taking prednisolone should be aware of the potential risks, including hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections. 1
Pharmacokinetics
Prednisolone is an oral medication with a bioavailability of approximately 70%. It reaches its peak concentration (Cmax) in the blood within 1.0-2.6 hours after administration, with a concentration range of 113-1343ng/mL. The volume of distribution for prednisolone varies depending on the dose administered, with a 0.15mg/kg dose having a volume of distribution of 29.3L and a 0.30mg/kg dose having a volume of distribution of 44.2L. Prednisolone's protein binding ranges from 65-91% in healthy patients. Metabolism of prednisolone involves reversible conversion to prednisone, which is then further metabolized to various metabolites such as M-XVII, M-V, M-XII, M-XIII, and M-IV. These metabolites undergo further conversion to other compounds, eventually leading to glucuronide conjugates that are predominantly excreted in the urine. Prednisolone itself is primarily eliminated in the urine, accounting for over 98% of the total elimination. The plasma half-life of prednisolone is approximately 2.1-3.5 hours, with variations observed in children and individuals with liver disease. The clearance rate of prednisolone is 0.09L/kg/h for a 0.15mg/kg dose and 0.12L/kg/h for a 0.30mg/kg dose. 2
Side effects
Prednisolone, when taken in excessive amounts, can lead to various side effects. Gastrointestinal disturbances, insomnia, and restlessness are common symptoms observed in patients experiencing an overdose of prednisolone. In cases of recent oral overdose, gastric lavage or inducing vomiting may be performed as initial measures, along with supportive and symptomatic therapy. Chronic overdosage can be managed by reducing the dose or adjusting the treatment schedule to alternate days. In animal studies, the lethal dose (LD50) of prednisolone has been determined. The intraperitoneal LD50 is 2g/kg in rats and 65mg/kg in mice. The subcutaneous LD50 is 147mg/kg in rats and >3500mg/kg in mice. Additionally, the oral LD50 in mice is 1680mg/kg. However, it is important to note that these findings are specific to animal models and may not directly translate to humans. In human studies, the oral TDLO (lowest published toxic dose) of prednisolone has been reported. For men, the oral TDLO is 9mg/kg/2W (two weeks), while for women, it is 14mg/kg/13D (thirteen days). These values indicate the lowest doses at which toxic effects have been observed in human subjects. It should be noted that an overdose of prednisolone administered through the ophthalmic route is not expected to cause problems. 3
Reference
1. Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. 2023 Jul 3. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
2. Pickup ME. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1979 Mar-Apr;4(2):111-128.
3. FDA Approved Drug Products: Prednisolone Acetate Oral Suspension, 1955.
You may like
Related articles And Qustion
Lastest Price from Prednisolone manufacturers

US $0.00-0.00/kg2025-07-08
- CAS:
- 50-24-8
- Min. Order:
- 1kg
- Purity:
- CEP/EP/USP
- Supply Ability:
- 10TONS

US $5.00-0.50/KG2025-05-08
- CAS:
- 50-24-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS