Cinnamic Acid: Dietary Sources, Mechanisms of Action and New Formulation
General Description
Cinnamic acid are widely present in plant-based foods, such as fruits, vegetables, whole grains, and coffee. It is easily absorbed from the small intestine and have potential mechanisms of action in stimulating insulin secretion, which may benefit individuals with diabetes. However, its limited bioavailability and weak biological effects have prompted the development of new formulations. These include self-nanoemulsifying drug delivery systems (SNEDDS), nanoparticles, nanostructured lipid carriers (NLCs), and solid lipid nanoparticles (SLNs). These new formulations aim to enhance the bioavailability and effectiveness of cinnamic acid for improved prevention and treatment of chronic diseases. Further research and clinical evaluation are necessary to fully explore their potential benefits.

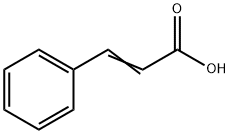
Figure 1. Cinnamic acid
Dietary Sources
Polyphenols, including cinnamic acid and its derivatives, are widely present in various plant-based foods and play an essential role in human diets. Cinnamic acid and its derivatives are synthesized in plants through the shikimate pathway using phenylalanine and tyrosine as precursor molecules. Cinnamic acid and its derivatives can be found in fruits, vegetables, whole grains, and other plant-based foods. For example, ferulic acid, one of the most common derivatives, is abundant in cereal grains, rice, and wheat bran. Caffeic acid, another derivative, is primarily sourced from coffee. It can also be found in sweet potatoes and artichokes. Cinnamic acid itself can be obtained from cinnamon, citrus fruits, grapes, tea, cocoa, spinach, celery, and brassicas vegetables. Isoferulic acid is commonly found in Chinese propolis and Cimicifuga, an herbal medicine in oriental countries. P-hydroxycinnamic acid, or p-coumaric acid, is mainly derived from peanuts, basil, and garlic. P-methoxycinnamic acid is a component of medicinal plants such as Granny's Nightcap and Buerger's Figwort. Various studies have estimated the daily consumption of cinnamic acid and its derivatives in different populations. The main sources of hydroxycinnamic acids intake include coffee, fruits, fruit juices, nuts, seeds, vegetables, and cereal products. It has been observed that cinnamic acid and its derivatives are easily absorbed from the small intestine through mechanisms like passive diffusion, monocarboxylic acid transporters (MCTs), and carrier-mediated transport involving Na+-dependent pathways. In conclusion, cinnamic acid and its derivatives are widely distributed in the human diet, with plant-based foods being the primary dietary sources. 1
Potential Mechanisms of Action
Cinnamic acid have been studied for their potential role in stimulating insulin secretion. The regulation of elevated circulating glucose levels is primarily controlled by insulin secretion from pancreatic β-cells. Glucose enters the β-cells through glucose transporter 2 (GLUT2) and is then phosphorylated to glucose-6 phosphate by glucokinase. It is further metabolized through glycolysis and oxidation, generating ATP. This leads to the closure of ATP-sensitive K+ channels (KATP channels), causing membrane depolarization and activation of voltage-dependent Ca2+ channels (VDCCs). The increase in intracellular calcium concentration ([Ca2+]) triggers the release of insulin from vesicles to the plasma membrane. Several studies have investigated the insulin-secreting activity of cinnamic acid and its derivatives. It was found that the presence of para-hydroxy and meta-methoxy groups in the structure of cinnamic acid exhibited the highest insulin-secreting activity. Ferulic acid stimulated insulin secretion in both cell lines and animal models. Similarly, p-methoxycinnamic acid was shown to stimulate insulin release at basal glucose levels and enhance glucose-induced insulin secretion. This effect was attributed to an increase in Ca2+ influx through L-type Ca2+ channels without inducing membrane depolarization by closing KATP channels. Overall, cinnamic acid show potential as insulin-secreting compounds and may have anti-diabetic activity by improving glucose tolerance and insulin secretion. 2
New Formulation
A new formulation of cinnamic acid has been developed to address the limited bioavailability and weak biological effects of this compound and its derivatives. Previous studies have shown that low plasma concentrations of cinnamic acid are primarily the result of limited absorption, intensive metabolism, and fast elimination from circulation. To enhance the bioavailability and effectiveness of cinnamic acid, researchers have created new formulations that encapsulate cinnamic acid derivatives into solid and liquid particles. One example is the self-nanoemulsifying drug delivery system (SNEDDS), which was developed to improve the bioavailability and anti-diabetic action of cinnamic acid. In a rat model, the SNEDDS formulation exhibited a 2.5-fold higher maximum plasma concentration (Cmax) compared to a cinnamic acid suspension. However, both formulations showed similar efficacy in reducing blood glucose and cholesterol levels in diabetic rats. In another study, cinnamic acid nanoparticles were prepared using a liquid anti-solvent precipitate process, resulting in a 2.16-fold higher oral bioavailability than unencapsulated cinnamic acid. Similarly, nanostructured lipid carriers (NLCs) and solid lipid nanoparticles (SLNs) loaded with ferulic acid were found to have greater oral bioavailability compared to non-encapsulated ferulic acid. Furthermore, electrospinning techniques were employed to encapsulate ferulic acid within amaranth protein isolate (API) and pullulan ultrathin fibers. This encapsulation improved the antioxidant capacity of ferulic acid during simulated gastrointestinal digestion, suggesting that these formulations could be promising for future clinical evaluation. In conclusion, these new formulations of cinnamic acid and its derivatives aim to enhance their bioavailability and biological effects, potentially leading to improved prevention and treatment of chronic diseases. Further research and clinical evaluation are needed to fully explore the potential benefits of these formulations. 3
Reference
1. Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. T oxicol. 2014, 65, 185–195.
2. Adisakwattana, S.; Moonsan, P .; Yibchok-Anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 7838–7844.
3. Li, W.; Zhao, X.; Sun, X.; Zu, Y .; Liu, Y .; Ge, Y . Evaluation of antioxidant ability in vitro and bioavailability of trans-cinnamic acid nanoparticle by liquid antisolvent precipitate. J. Nanomater. 2016, 2016, 9518362.
You may like
Related articles And Qustion
Lastest Price from Cinnamic acid manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 621-82-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 25tons/month

US $6.00/kg2025-04-21
- CAS:
- 621-82-9
- Min. Order:
- 7kg
- Purity:
- 0.99
- Supply Ability:
- 10000