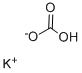
Potassium bicarbonate: The all-rounder of the chemical world
Introduction
With its wide range of applications, potassium bicarbonate has won wide attention in the scientific community. As an important inorganic salt, it not only occupies an important position in chemical production but also shows its applications in many fields, such as food processing, medicine, agriculture, and environmental protection. This article will introduce the application of potassium bicarbonate in human health and agriculture.

Human Health
Reduce Cardiovascular Risk Factors1
One study from Graham A. MacGregor and colleagues demonstrated that, in individuals who already had a relatively low salt and high potassium intake, supplementation of either potassium chloride or potassium bicarbonate improved endothelial function and reduced cardiovascular risk factors. Potassium bicarbonate also had beneficial effects on calcium and bone metabolism. The potassium intake achieved in this study is very similar to the adequate intake for adults recommended by the US Institute of Medicine (120 mmol/d). The current potassium intake in most populations is 60 to 70 mmol/d. Increasing potassium intake could play an important role in the prevention of cardiovascular disease and osteoporosis in the long term. Although this study was carried out in individuals with raised BP, it is likely that the general population would also benefit from an increase in potassium intake. This is reinforced by the findings from prospective studies in general populations that a higher potassium intake was related to a lower risk of cardiovascular disease.
A randomized trial in 1981 elderly Taiwanese demonstrated that replacing the usual salt for a potassium-enriched salt, with a subsequent increase of 76% in potassium intake and a 17% reduction in salt intake, resulted in a 40% reduction in cardiovascular disease mortality. This study provides further support for an increase in population potassium intake, and this would best be done by an increase in the consumption of foods high in potassium, for example, fruit and vegetables that in themselves have beneficial effects on health independent of potassium intake.
Improve Calcium and Phosphorus Balance2
In normal subjects, a low level of metabolic acidosis and positive acid balance (the production of more acid than is excreted) are typically present and correlate in degree with the amount of endogenous acid produced by the metabolism of foods in ordinary diets abundant in protein. Over a lifetime, the counteraction of retained endogenous acid by base mobilized from the skeleton may contribute to the decrease in bone mass that occurs normally with aging.
To test that possibility, R C Morris Jr and colleagues administered potassium bicarbonate to 18 postmenopausal women who were given a constant diet (652 mg [16 mmol] of calcium and 96 g of protein per 60 kg of body weight). The potassium bicarbonate was given orally for 18 days in doses (60 to 120 mmol per day) that nearly completely neutralized the endogenous acid.
The result showed that, in postmenopausal women, the oral administration of potassium bicarbonate at a dose sufficient to neutralize endogenous acid improves calcium and phosphorus balance, reduces bone resorption, and increases the rate of bone formation.
Agriculture3
Bicarbonate salts are widely used in the food industry and have broad-spectrum antimicrobial activity. E.Fallir and colleagues investigated whether bicarbonate salts can serve as alternatives to synthetic chemical fungicides for controlling postharvest diseases of fruits and vegetables. Potassium bicarbonate (PBC) inhibited in vitro mycelial growth, spore germination and germ tube elongation of Botrytis cinerea and Alternaria alternata. Germ tube elongation was found to be more sensitive to elevated PBC concentrations than mycelial growth and spore germination, as measured by EC50. Treatment with PBC resulted in shrinkage and collapse of hyphae and spores, and consequent inability of fungi to sporulate. PBC action was fungistatic rather than fungicidic. Dipping commercially harvested sweet red peppers for 2 min in 1 or 2% potassium bicarbonate reduced decay development after storage and shelf life simulation to a commercially acceptable level of 5–8% compared with untreated or water-dipped controls. Higher concentrations of PBC (3%) significantly reduced fruit quality.
References:
[1] FENG J HE. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives.[J]. Hypertension, 2010, 55 3. DOI:10.1161/HYPERTENSIONAHA.109.147488.[2] A SEBASTIAN R C M. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate.[J]. New England Journal of Medicine, 1994, 331 4. DOI:10.1056/NEJM199407283310421.
[3] E. FALLIK O Z S Grinberg. Potassium bicarbonate reduces postharvest decay development on bell pepper fruits[J]. Journal of Horticultural Science, 1997, 42 1: 525-526. DOI:10.1080/14620316.1997.11515489.
You may like
Related articles And Qustion
Lastest Price from Potassium bicarbonate manufacturers

US $1200.00-1100.00/ton2025-11-07
- CAS:
- 298-14-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $10.00/KG2025-04-21
- CAS:
- 298-14-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt