Polycarbonate: A Cornerstone of Modern Material Science
Introduction
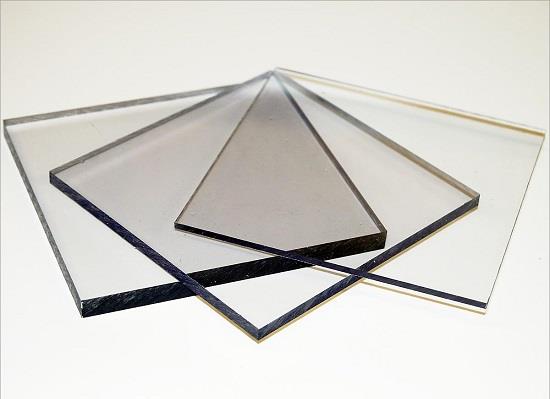
Polycarbonate, a marvel of modern chemistry, stands out as a superior material in multiple domains owing to its unique blend of properties. This thermoplastic polymer, renowned for its resilience and optical clarity, has been widely adopted across diverse industries, including automotive, electronics, construction, and medical technology. Its impact resistance, coupled with its ability to transmit light nearly as well as glass, makes it an ideal choice for applications requiring robust, transparent materials. These characteristics also facilitate its use in safety equipment, such as bulletproof windows and eyewear lenses, enhancing both functionality and safety in critical applications.

Figure 1 Characteristics of Polycarbonate
Composition and Mechanism of Action
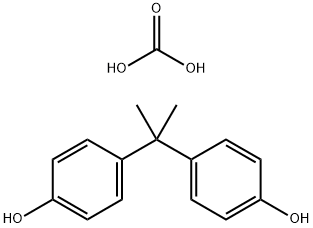
Polycarbonate's chemical structure is primarily based on bisphenol A (BPA) linked through carbonate groups (-O-(C=O)-O-). This arrangement is achieved through a synthesis process known as condensation polymerization, where BPA and phosgene react to form a long-chain polymer. The polycarbonate produced through this method exhibits not only a high degree of transparency but also an exceptional level of impact resistance and thermal stability.
At a molecular level, polycarbonate's performance is attributable to its unique structural properties. The bisphenol A units contribute rigidity, while the carbonate linkages introduce flexibility. This balance is crucial for maintaining the material’s mechanical strength across a broad temperature range. Additionally, the aromatic rings present in its structure serve to absorb UV radiation, thereby mitigating the risk of polymer degradation when exposed to sunlight. This property is particularly valued in applications requiring long-term durability in harsh environmental conditions.
Applications of Polycarbonate
Polycarbonate's robust and versatile nature has led to its utilization in a myriad of applications that capitalize on its unique properties. In the automotive sector, it is employed in the production of lightweight yet durable parts such as windows, sunroofs, and protective headlamp covers. These applications benefit from the material’s resistance to impact and ability to withstand various weather conditions without significant degradation[2].
In the realm of safety and security, polycarbonate is an indispensable material in the production of bulletproof glass and protective gear, including helmets and shields used by law enforcement. Its high-impact resistance ensures maximum protection against physical threats, making it an essential component of personal protective equipment.
The use of polycarbonate extends into the electronics industry, where it is integral to the manufacturing of smartphone cases, laptops, and other devices that require a high degree of durability along with electrical insulation. Its resistance to heat and electrical insulation properties make it ideal for protecting sensitive electronic components from environmental stress and operational heat.
Storage Guidelines for Polycarbonate
Effective storage of polycarbonate is critical to preserve its physical and mechanical qualities. It is recommended to store polycarbonate sheets and components in a cool, dry place, shielded from UV light to prevent premature aging. If exposed to excessive sunlight, the material may undergo yellowing and a reduction in impact strength over time.
For industrial settings where polycarbonate pellets are used, maintaining a controlled environment is vital. The pellets should be kept in moisture-proof containers to avoid absorption of moisture that can lead to hydrolysis during the molding process, thereby degrading the polymer's properties. It is also advisable to avoid storing polycarbonate near chemicals that could emit fumes, as these might affect the polymer's structural integrity.
Conclusion
Polycarbonate continues to be a material of significant interest and utility in modern material science due to its outstanding properties and adaptability. Its applications are vast and continue to expand as new technologies and manufacturing processes evolve. The ongoing research and development in polymer chemistry are likely to enhance its capabilities even further, promising innovations and applications that could redefine what is possible with this versatile material.
References
[1]Davis, A., and J. H. Golden. "Stability of polycarbonate."Journal of Macromolecular Science, Part C3.1 (1969): 49-68.
[2]Antonakou, E. V., and D. S. Achilias. "Recent advances in polycarbonate recycling: A review of degradation methods and their mechanisms."Waste and Biomass Valorization4 (2013): 9-21.
Related articles And Qustion
Lastest Price from Polycarbonate manufacturers
US $0.00-0.00/KG2025-11-18
- CAS:
- 25037-45-0
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $10.00/KG2025-04-21
- CAS:
- 25037-45-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt