Poly(propylene glycol) bis(2-aminopropyl ether): A Review of Synthesis, Properties, Applications, and Environmental Considerations
Introduction
Poly(propylene glycol) bis(2-aminopropyl ether), though not as widely recognized by its full name, plays a pivotal role in diverse fields due to its distinct chemical characteristics. It belongs to a class of compounds known for their ability to act as intermediates or components in the production of polyurethanes, coatings, and adhesives. Understanding its synthesis, properties, and applications provides insights into its versatility and potential for further development.
Synthesis and Chemical Structure
The synthesis of poly(propylene glycol) bis(2-aminopropyl ether) involves a series of controlled chemical reactions. Typically, it starts with propylene oxide, which undergoes polymerization to form poly(propylene glycol) (PPG). This polymerization reaction is catalyzed under specific conditions to achieve the desired chain length and viscosity of the PPG. Subsequently, the PPG is reacted with an appropriate amine-containing compound, such as 2-aminopropyl ether, to introduce the amino functional groups at both ends of the polypropylene glycol chain. This reaction is carefully controlled to ensure the formation of the bis(2-aminopropyl ether) derivative, which is crucial for its subsequent applications.
The chemical structure of poly(propylene glycol) bis(2-aminopropyl ether) is characterized by a central poly(propylene glycol) backbone with two amino-terminated side chains. This structure confers unique properties, including good solubility in organic solvents, reactivity towards isocyanates, and the ability to form strong intermolecular bonds. These properties make it an attractive choice for various chemical formulations.1
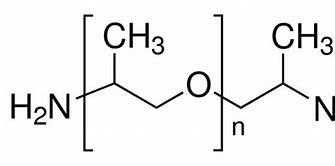
Physical and Chemical Properties
Poly(propylene glycol) bis(2-aminopropyl ether) exhibits a range of physical and chemical properties that contribute to its versatility in applications. It is a viscous liquid at room temperature, with its viscosity varying depending on the molecular weight of the polypropylene glycol backbone. This characteristic allows for tailor-made formulations with specific rheological properties.
Chemically, it is stable under normal storage conditions but can react with various compounds, especially isocyanates, to form polyurethanes. Its amino groups are highly reactive, enabling it to participate in a wide array of chemical reactions, including crosslinking and chain extension. Additionally, it possesses good compatibility with other organic compounds, making it suitable for use in complex mixtures.2
Applications
The versatility of poly(propylene glycol) bis(2-aminopropyl ether) is evident in its wide range of applications. One of its primary uses is in the production of polyurethanes. As a chain extender or crosslinker, it enhances the mechanical properties of polyurethanes, such as tensile strength and elasticity. This makes it a valuable component in the manufacture of flexible foams, elastomers, and coatings.3
In the coatings industry, poly(propylene glycol) bis(2-aminopropyl ether) is used to modify the properties of resins and polymers. It can improve the adhesion, durability, and chemical resistance of coatings, making them suitable for applications in automotive, construction, and industrial settings.
Furthermore, its ability to react with isocyanates has led to its utilization in adhesive formulations. It enhances the bonding strength and flexibility of adhesives, making them ideal for bonding diverse materials, including metals, plastics, and composites.
Poly(propylene glycol) bis(2-aminopropyl ether) also finds applications in personal care and cosmetic products due to its emollient properties and ability to improve the texture of formulations. It is used in skincare products, hair care products, and makeup to enhance spreadability, moisturization, and overall product performance.
Environmental Considerations
As with any chemical compound, the environmental impact of poly(propylene glycol) bis(2-aminopropyl ether) must be considered. While it offers numerous benefits in various applications, its production and disposal can have environmental consequences if not managed properly.
The synthesis of poly(propylene glycol) bis(2-aminopropyl ether) involves the use of chemicals and energy, which can contribute to greenhouse gas emissions and waste generation. However, advancements in synthesis techniques and the adoption of sustainable practices can mitigate these impacts.
In terms of its end-of-life management, when incorporated into polyurethanes or other formulated products, can be challenging to recycle. This underscores the importance of developing effective recycling and disposal strategies to minimize waste and environmental contamination.4
Future Perspectives
The ongoing research and development in the field of poly(propylene glycol) bis(2-aminopropyl ether) and its derivatives hold promise for further innovations. Ongoing efforts focus on enhancing its properties, such as improving its reactivity, solubility, and compatibility with renewable materials. This could lead to the development of more sustainable and eco-friendly formulations.
Moreover, the exploration of new applications, particularly in emerging fields such as renewable energy and advanced materials, presents exciting opportunities. As the demand for high-performance and environmentally conscious materials grows, poly(propylene glycol) bis(2-aminopropyl ether) could play a pivotal role in meeting these demands.5
Conclusion
Poly(propylene glycol) bis(2-aminopropyl ether), despite its complex name, is a remarkable compound with a wide array of applications. Its unique chemical structure, coupled with its reactive amino groups, confers versatility and functionality in various industries. From polyurethanes to coatings and adhesives, its contributions are significant and wide-ranging.
However, the use of this compound must be balanced with environmental considerations. As research and development continue, it is crucial to explore sustainable synthesis methods, effective waste management strategies, and new applications that align with environmental goals. By doing so, poly(propylene glycol) bis(2-aminopropyl ether) can continue to serve as a valuable component in advancing technology and industry while minimizing its ecological footprint.
References:
[1] LIN GU J H Yuanzhang Jiang. Synthesis and Properties of Shape Memory Poly(γ-Benzyl-l-Glutamate)-b-Poly(Propylene Glycol)-b-Poly(γ-Benzyl-l-Glutamate)[J]. Applied Sciences-Basel, 2017, 7 1: 1258. DOI:10.3390/APP7121258.[2] CHUNWON LIM H K Suk In Hong. Effect of polyether diamine on gas permeation properties of organic-inorganic hybrid membranes[J]. Journal of Sol-Gel Science and Technology, 2007, 43 1. DOI:10.1007/s10971-007-1553-7.
[3] KOZO MATSUMOTO T E. Synthesis of Ion Conductive Networked Polymers Based on an Ionic Liquid Epoxide Having a Quaternary Ammonium Salt Structure[J]. Macromolecules, 2009, 42 13: 4353-4920. DOI:10.1021/ma900508q.
[4] ALINA ANGHELACHE. Novel crosslinked thermoresponsive hydrogels with controlled poly(ethylene glycol)—poly(propylene glycol) multiblock copolymer structure[J]. Colloid and Polymer Science, 2013, 292 4. DOI:10.1007/s00396-013-3128-1.
[5] MAGDALENA TARNACKA*. The Impact of Molecular Weight on the Behavior of Poly(propylene glycol) Derivatives Confined within Alumina Templates[J]. Macromolecules, 2019, 52 9: 3151-3574. DOI:10.1021/acs.macromol.9b00209.
See also
Lastest Price from Poly(propylene glycol) bis(2-aminopropyl ether) manufacturers

US $10.00/KG2025-06-26
- CAS:
- 9046-10-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $1500.00/KG2025-05-08
- CAS:
- 9046-10-0
- Min. Order:
- 1KG
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min