Pivalic Acid: Effect and Reactivity
Introduction
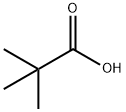
Pivalic acid is a very important chemical raw material and reagent, as well as an important raw material and intermediate for organic synthesis. Pivalic acid It is a white needle-shaped crystal at room temperature, with a melting point of 35.5 ℃ and a boiling point of 163.8 ℃. Its density (50 ℃) is 0.905g/cm3, and it is easily soluble in alcohols and ethers, but not easily hydrolyzed. Pivalic acid can be used as a stabilizer for polyvinyl chloride and other paints, as well as a free radical promoter.

Figure 1 Characteristics of Pivalic acid
Effect
Pivalic acid As a co-catalyst of palladium, it is used for the arylation reaction of unactivated aromatic hydrocarbons and N-heterocycles.
Pivalic acid As an additive, palladium nanoparticles are used as catalysts to promote the carbonylation Suzuki reaction and to synthesize aryl ketones from aryl iodides and aryl boronic acids.
Pivalic acid In the cyclization reaction between benzamide and alkynes, isoquinolones are synthesized in the presence of 8-aminoquinoline ligand and cobalt catalyst.
Reactivity Profile
Pivalic acid is a carboxylic acid. If there is a base to accept carboxylic acid, the carboxylic acid will provide hydrogen ions. They react with all bases in this way, including organic bases (such as amines) and inorganic bases. Their reaction with alkali is called "neutralization", accompanied by the release of a large amount of heat. The neutralization between acid and alkali produces water and salt. Carboxylic acids with 6 or fewer carbon atoms, are freely or moderately soluble in water; Those with carbon numbers exceeding 6 are slightly soluble in water. Soluble carboxylic acids dissociate to a certain extent in water, generating hydrogen ions. Therefore, the pH value of the carboxylic acid solution is less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing chemical bases and dissolve upon neutralization to form soluble salts. Carboxylic acids in aqueous solutions and liquid or molten carboxylic acids can react with active metals to generate gaseous hydrogen and metal salts. This reaction principle also applies to solid carboxylic acids, but if the solid acid remains dry, the reaction will be very slow. Even 'insoluble' carboxylic acids can absorb enough water from the air and fully dissolve in valeric acid, thereby corroding or dissolving iron, steel, and aluminum components and containers. Carboxylic acids, like other acids, react with cyanide salts to produce gaseous hydrogen cyanide. For dry solid carboxylic acids, the reaction is slower. Insoluble carboxylic acids react with cyanide solutions, releasing gaseous hydrogen cyanide. Carboxylic acids react with diazo compounds, dithiocarbamate, isocyanates, thiols, nitrides, and sulfides to generate flammable and/or toxic gases and heat. Carboxylic acids, especially in aqueous solutions, also react with sulfites, nitrites, thiosulfates (generating H2S and SO3), and disulfides (SO2) to produce flammable and/or toxic gases and heat. They react with carbonates and bicarbonates to produce harmless gases (carbon dioxide) but still generate heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidants and reduced by strong reducing agents. These reactions generate heat. There are many types of products. Like other acids, carboxylic acids may trigger polymerization reactions; Like other acids, they often catalyze (increase) chemical reactions.
Security information
Pivalic acid has strong irritant and corrosive properties, and contact with the skin and eyes can cause serious chemical burns. Proper protective equipment should be worn during operation to avoid direct contact. Tevaleric acid is also a flammable liquid, and fire and explosion prevention measures should be taken during storage and use. Tevaleric acid should be kept away from sources of fire, heat, and oxidants, and stored in a cool, well-ventilated place. When using Tevaleric acid, please be sure to follow the relevant safety operating procedures and store, handle, and dispose of the chemical properly.
References:
[1] MARC LAFRANCE K F. Palladium-Catalyzed Benzene Arylation: Incorporation of Catalytic Pivalic Acid as a Proton Shuttle and a Key Element in Catalyst Design[J]. Journal of the American Chemical Society, 2006, 128 51: 16414-17157. DOI:10.1021/ja067144j.[2] E. R. RUBINSTEIN M G. Dendritic grown kinetics and structure I. Pivalic acid[J]. Journal of Crystal Growth, 1991, 112 1: 84-96. DOI:10.1016/0022-0248(91)90914-Q.
See also
Lastest Price from Pivalic acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 75-98-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $0.00/kg2025-04-18
- CAS:
- 75-98-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000MT