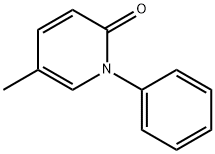
Pirfenidone: uses, Mechanism of Action, and Side effects
What is Pirfenidone?
Pirfenidone, an orally active small molecule, is an antifibrotic agent with anti-TGF-β and anti-TNF properties. It inhibits the production of profibrotic and inflammatory cytokines (TGFb, PDGF, IL-6, and TNF), blocks myofibroblast differentiation and fibroblast proliferation, and collagen production. However, its precise mechanism of action is incompletely understood. It is a scavenger of reactive oxygen species (ROS) at high concentrations.
What are the uses of Pirfenidone?
Pirfenidone (Esbriet®) is an orally administered, synthetic, pyridone compound that is approved for the treatment of adults with mild to moderate idiopathic pulmonary fibrosis (IPF) in the EU and the treatment of IPF in the USA.
In rats with anti-GBM glomerulonephritis, both candesartan and Pirfenidone decreased proteinuria and glomerulosclerosis, and the combination of both drugs showed additive effects. Pirfenidone reduces interstitial fibrosis in the remnant nephron rat model but does not reduce proteinuria. Similarly, it reduces mesangial matrix expansion in db/db mice without affecting proteinuria. In a one-arm trial involving 21 patients with idiopathic and postadaptive FSGS, pirfenidone treatment slowed the GFR decline rate but did not affect proteinuria. Thus, Pirfenidone holds promise as an antifibrotic drug but seems to lack a consistent antiproteinuric effect.
What is Pirfenidone's Mechanism of Action?
Uncertainty surrounds the precise mechanism of action of Pirfenidone. However, it is believed to have antioxidant, anti-inflammatory, and antifibrotic properties. It is used as a drug to treat Idiopathic pulmonary fibrosis (IPF) because it is thought to inhibit fibroblast growth, their transformation into myofibroblasts, and the generation of collagen, demonstrating some antifibrotic efficacy. It was observed that the patients treated with Pirfenidone showed more than a 10% decline in FVC than the patients treated with placebo after following up after 52 weeks. Even with treatment-emergent adverse events (TEAEs), the discontinuation of the treatment was low.
The disease progression events assessed in the study were a % decline in FVC and a six-minute walking distance (6MWD). It was observed that the patients who received Pirfenidone had more than one progression event. Deaths reported were fewer compared to the placebo group. It was observed that Pirfenidone reduced the episodes of dyspnea in patients who showed FVC < 80% or GAP stage II/III. The benefit of dyspnea reduction was not observed much in patients with preserved FVC and GAP stage I. Pirfenidone showed efficacy in reducing the decline in lung function after its initiation, but it was not able to restore the already damaged function of the lung. Thus, the timing for initiating the treatment is beneficial to the patient with IPF, irrespective of the stage of IPF.
Side effects
Adverse effects are mostly gastrointestinal (nausea, dyspepsia, anorexia, gastroesophageal reflux). Skin-related complications are less frequent (e.g., photosensitivity rash). Alanine or aspartate aminotransferase levels are frequently elevated during treatment with Pirfenidone, but this is reversible and has no clinically significant effect.
[1] Esther S Kim, Gillian M Keating. “Pirfenidone: a review of its use in idiopathic pulmonary fibrosis.” Drugs 75 2 (2015): 219–30.
[2] Ruzhual K Man. “A Comparison of the Effectiveness of Nintedanib and Pirfenidone in Treating Idiopathic Pulmonary Fibrosis: A Systematic Review.” Cureus 16 2 (2024): e54268.
[3] Pirfenidone - an overview | ScienceDirect Topics https://www.sciencedirect.com/topics/medicine-and-dentistry/pirfenidone
References:
[1] ESTHER S KIM G M K. Pirfenidone: a review of its use in idiopathic pulmonary fibrosis.[J]. Drugs, 2015, 75 2. DOI:10.1007/s40265-015-0350-9.[2] RUZHUAL K MAN. A Comparison of the Effectiveness of Nintedanib and Pirfenidone in Treating Idiopathic Pulmonary Fibrosis: A Systematic Review.[J]. Cureus, 2024, 16 2. DOI:10.7759/cureus.54268.
You may like
Related articles And Qustion
Lastest Price from Pirfenidone manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 53179-13-8
- Min. Order:
- 1KG
- Purity:
- 98.5%-101%
- Supply Ability:
- 100KG

US $100.00-75.00/kg2025-04-21
- CAS:
- 53179-13-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000