Photochemical behaviour of phenylurea herbicides
Description
Phenylurea herbicides are a group of pesticides used for general weed control in agricultural and nonagricultural practices"for example, along railroads, utilities, rights- of-way, and in industrial areas. These are now manufactured and distributed under the names of anisuron, buturon, chlorbromuron, chlortoluron, chloroxuron, difenoxuron, diuron, fenuron, fluometuron, isoproturon, linuron, methiuron, metobromuron, metoxuron, monuron, neburon, parafluron, siduron, tebuthiuron, tetrafluron, and thidiazuron.
The herbicidal action of these compounds is based on their ability to inhibit photosynthesis. Typical phenylurea herbicides are photosystem II inhibitors.
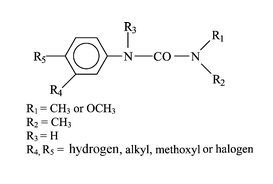
Degradation of phenylurea herbicides
Degradation of phenylurea herbicides in nature can be a relatively slow process. These pesticides can be decomposed by UV irradiation or by acidic or alkaline conditions. There are four basic types of reactions in the photochemistry of the substituted phenylurea herbicides: photolysis of the C-X bond on aromatics, photoeliminations, photooxidations, and photorearrangements.
Although approximately 20 different phenylureas have been marketed, there is relatively little toxicity information available for most. Diuron, the most common phenylurea, has been among the top 10 pesticides in use in the United States. The acute toxicity potential for all phenylureas appears to be low, with oral LD 50 values typically greater than 1 g/kg.
Photochemical behaviour
The photochemical behaviour of phenylurea herbicides in aqueous solution is highly dependent on the nature and position of substituents on the ring. Most of these herbicides are methylated on the urea moiety, the other substituents are usually halogens or methoxy groups. The main reaction involving the aromatic ring of unhalogenated phenylureas excited at wavelengths shorter than 300 nm is an intramolecular rearrangement, similar to photo-Fries rearrangement, whereas with halogenated derivatives, photohydrolysis is the main transformation pathway.
In the particular case of para-halogenated phenylureas, the intermediate formation of a carbene is observed. When the urea moiety is substituted with a methoxyl group, demethoxylation is a competitive reaction. N-Demethylation or oxidation of methyl groups is also observed, but with a lower yield.
Photooxidation of phenylureas can also be induced by photocatalysis, iron salts or humic substances. In the absence of water, the main route for phototransformation of diuron is the oxidation or elimination of methyl groups. It is entirely possible that a photochemical intermediate could turn out to be more toxic than the initial herbicide.
You may like
Related articles And Qustion
Lastest Price from Phenylurea manufacturers

US $10.00/kg2025-09-02
- CAS:
- 64-10-8
- Min. Order:
- 25kg
- Purity:
- >99.9%
- Supply Ability:
- 200tons

US $0.00-0.00/KG2025-08-18
- CAS:
- 64-10-8
- Min. Order:
- 1KG
- Purity:
- 99%Min
- Supply Ability:
- 3000 tons