Pharmacokinetics of quinine in obesity
Introduction
Quinine is a natural cinchona alkaloid that has been used for centuries in the prevention and therapy of malaria.Quinine acts against the asexual erythrocytic forms of malaria, including Plasmodium vivax, malariae and falciparum and is gametosidal to P. vivax and malariae. The use of quinine for malaria has been largely replaced by chloroquine, which is more potent and better tolerated.Quinine acts against the asexual erythrocytic forms of malaria, including Plasmodium vivax, malariae and falciparum and is gametosidal to P. vivax and malariae. The use of quinine for malaria has been largely replaced by chloroquine, which is more potent and better tolerated.Quinine is also used for idiopathic muscle cramps.Quinine is also used for idiopathic muscle cramps[1].In addition,the potential of quinine sulfate (QS) for COVID-19 treatment, among others, has the same basic structure with chloroquine (CQ) and hydroxychloroquine (HCQ) , namely Quinoline, which can inhibit viral fusion; is weakly alkaline so that it can increase the pH of cell organelles; has higher binding affinity to SARS-CoV-2 compared with CQ and HCQ; has antiviral activity against SARS-CoV-2 in-vitro; has other antiviral activity and acts as an immunomodulator[2]. Common side effects of quinine include headache, dizziness, blurred vision, gastrointestinal upset, thrombocytopenia and hypersensitivity reactions[1].Today we present a literature to understand the characteristics of quinine in obese people[3].
Obesity is common, especially in the USA where the prevalence of obesity has increased from 25.4% in 1976-80 to 33.3% in 1988-91[4]. Current drug dosing recommendations are usually based on published pharmacokinetic data from studies in subjects or patients of normal bodyweight. These recommendations may be inaccurate when applied to obese patients owing to physiological changes of obesity, which affect the pharmacokinetics of drugs [5]. Many studies have shown that obesity affects the pharmacokinetics and metabolism of highly lipid-soluble drugs[5-6]. As quinine is a lipophilic drug and has low hepatic clearance with a narrow therapeutic index, significant alterations in the pharmacokinetics of quinine in obese individuals might have clinical implications with respect to dosage regimens in obese patients. Therefore, the present study was conducted to determine the effects of obesity on the pharmacokinetics of quinine after a single oral dose of quinine.
Subjects
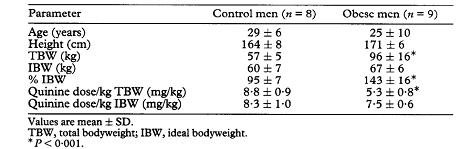
Nine obese men (aged 18-49 years) and 8 male lean (control) volunteers (aged 19-41 years) participated in the study. All subjects gave written informed consent. They were non-smoking Thai healthy volunteers. The studied men had no history of drug hypersensitivity and had not taken any medication for 2 weeks before entering and during the study. They were determined to be healthy by medical history, physical examination and blood chemistry tests. Ideal bodyweight (IBW) was defined as: IBW (male) = 49.9 kg+0.89 kg/cm above 152.4 cm height. The percentage IBW (% IBW) was calculated as the ratio of total bodyweight to IBW. Subjects were classified as obese if their total bodyweight was 125% or more of IBW. Mean % IBW ± standard deviation (SD) was 143±16 in the obese subjects, range 123-169, and 95±7 in the controls (range 88-110, Table 1). Owing to difficulty in the recruitment of obese subjects, 1 volunteer with 123% IBW was included in the obese group.
Study design
After an overnight fast, all subjects received an oral dose of 600 mg quinine sulphate with a glass of water. Quinine sulphate tablets (Quinoc®300 mg) were kindly supplied by Douglas Pharmaceutical, Auckland, New Zealand. Subjects remained fasting for 3 h after drug administration.Venous blood samples were collected into heparinized tubes before the dose, and at 0.25,0.5,0.75,1,1.5,2,2.5,3,4,6,8,10,12,24,30,36 and 48 h after dosing. Blood samples were centrifuged, and the plasma was separated. The plasma samples were kept frozen at -20°C until assayed.
Results
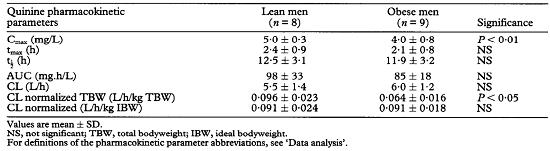
Statistical analysis of the subjects’demographics did not reveal significant differences between obese and lean (control) subjects according to age, height and IBW (Table 1). Mean total bodyweight (TBW) of the obese group was significantly greater than that in the controls (96±16 kg vs 57±5 kg, P< 0.001). The TBW of obese subjects was 143±16% of the IBW, whereas that of control subjects was 95±7% of IBW. Because the obese subjects weighed more than the control individuals, the doses of quinine administered to this group were significantly less than to the controls, on a milligram per kilogram TBW basis (Table 1). In contrast, the quinine doses based on a milligram per kilogram IBW were not significantly different between the 2 groups.
Figure 1 is a plot of the mean plasma quinine concentrations as a function of time in obese and control groups. In both groups, peak plasma concentrations (Cmax) of quinine were rapidly achieved within approximately 2 h and a monoexponential decline of concentrations in the terminal (elimination) phase was observed.
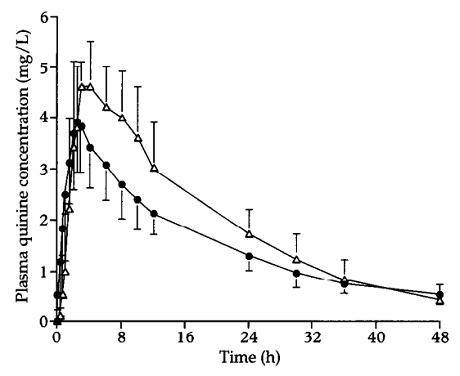
Table 2 lists the derived pharmacokinetic parameters of quinine in the 2 groups. Mean Cmax in the obese subjects was significantly lower than that observed in the controls (4.0±0.8 mg/L vs 5.0±0.3 mg/L, P< 0.01). Mean values of tmax,half-life (t1/2) and total AUC (AUC0-∞) were similar between the 2 groups. Absolute CL of quinine in the obese group was not significantly different from that observed in the control group (Table 2). When CL was normalized by the TBW, there was a significant difference (P < 0.05) in this parameter between the obese and the control groups. However, when CL was normalized by IBW, the difference between the 2 groups was not statistically significant (Table 2).
Discussion
The present study showed that the dosage of quinine based on TBW in the control (lean) group (8.8±0.9 me/kg. Table 1) is similar to the usual recommended maintenance dose of quinine in adults, 10 mg/kg [7]. In contrast, the mean quinine dose based on TBW in the obese group (5.3±0.8 mg/kg, Table 1) was significantly less than that given to the controls (P < 0.001). If this maintenance dose is used in obese individuals, in terms of mg/kg TBW, clinicians may respond by increasing the dosage of quinine according to their TBW. Such a recommendation would be wrong because the results from the present study have shown that the mean total (absolute) clearances of quinine are similar for the obese and the control groups (6.0±1.2 vs 5.5±1.4 L/h, P > 0.05, Table 2). In fact, the clearance of quinine in the obese group, when normalized to the IBW, was not significantly different from that observed in the control group. The similarity of clearance values between the 2 groups indicates that the ability of the liver to eliminate quinine is not affected by obesity. Therefore, the maintenance dose of quinine for obese patients should be given on the basis of patient's IBW, not based on the TBW.
In summary, on the basis of this information, the present study recommend that the maintenance dose of quinine in obese patients be based on ideal bodyweight, not total bodyweight. Otherwise, unnecessary adverse drug reactions may occur if the maintenance dose is given according to obese patients’total bodyweight.
References
[1] Quinine. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; May 15, 2018.
[2] Latarissa IR, Barliana MI, Meiliana A, Lestari K. Potential of Quinine Sulfate for COVID-19 Treatment and Its Safety Profile: Review. Clin Pharmacol. 2021;13:225-234.
[3] Viriyayudhakorn S. , Thitiarchakul S. , Nachaisit S, et al. Pharmacokinetics of quinine in obesity.[J]. Transactions of the Royal Society of Tropical Medicine and Hygiene,2000,94(4):425-428.
[4] Kuczmarski R J , Flegal K M , Campbell S M ,et al.Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991[J].JAMA The Journal of the American Medical Association, 1994, 272(3):205-211.
[5] Cheymol G .Clinical Pharmacokinetics of Drugs in Obesity[J].Clinical Pharmacokinetics, 1993, 25(2):103-114.
[6] Kotlyar M, Carson S W .Effects of obesity on the cytochrome P450 enzyme system.[J].International Journal of Clinical Pharmacology & Therapeutics, 1999,37(1):8-19.\
[7] White N J , Chanthavanich P , Krishna S ,et al.Quinine disposition kinetics.[J].British Journal of Clinical Pharmacology, 1983,16:399-403.
You may like
Related articles And Qustion
See also
Lastest Price from Quinine manufacturers

US $0.00-0.00/kg2025-10-17
- CAS:
- 130-95-0
- Min. Order:
- 0.1kg
- Purity:
- 99.9
- Supply Ability:
- 2000kgs/month

US $10.00/ASSAYS2025-05-04
- CAS:
- 130-95-0
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton