Pharmacokinetic and bioequivalence study of cefprozil suspension granules
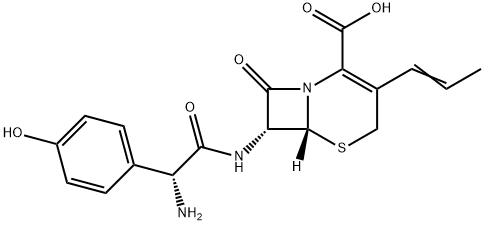
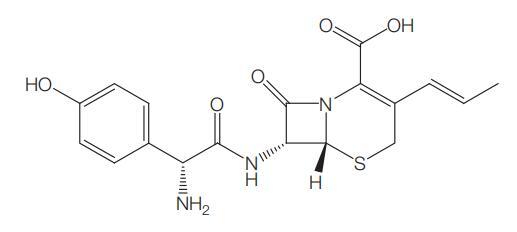
Introduction of Cefprozil
Cefprozil is an oral second-generation semisynthetic cephalosporin with broad-spectrum antibacterial activity. It is used to treat certain infections caused by bacteria, such as bronchitis (infection of the airway tubes leading to the lungs), as well as infections of the skin, ears, sinuses, throat and tonsils. It works by stopping the growth of bacteria. However, this medication is not used for colds, flu, or other viral infections.

Cefprozil granules vs. dry suspension
Cefprozil is administered orally in the form of tablets and suspensions (liquids). A granular formulation has been developed to improve patient adherence, and a bioequivalence assessment of granules versus dry suspension in Chinese healthy volunteers was conducted to estimate the pharmacokinetic (PK) profile of cefprozil.
Methods: An open-label, randomized, single-dose, two-period, two-group, crossover study was conducted in 60 healthy Chinese volunteers under fasted or fed conditions (30 volunteers for each condition) to assess bioequivalence between two formulations cefprozil. Blood samples were collected at specified time intervals, plasma concentrations cis- trans-cefprozil were determined by a validated liquid chromatography-tandem mass spectrometry (LC–MS/MS) method. PK bioavailability parameters were estimated via non-compartmental methods. Adverse events (AEs) were also recorded.
Results: Under fasted conditions, mean Cmax was (3534.70 ± 634.67) ng/ml, Tmax was (0.98 ± 0.25) h, t1/2 was (1.37 ± 0.13) h AUC0-t was (9302.86 ± 1618.39) ng·h/ml, respectively, after a single dose 125 mg cefprozil for suspension. Under fed conditions, mean Cmax was (2438.80 ± 493.78) ng/ml, Tmax was (1.66 ± 0.76) h, t1/2 was (1.36 ± 0.24) h AUC0-t was (9332.36 ± 1373.61) ng·h/ml, respectively. The PK parameters granule formulation cefprozil were similar to those suspension. The 90% CI values GMRs Cmax, AUC0-t AUC0-∞ under both fasted fed conditions were within prespecified bioequivalence range (80.00–125.00%).
According to criteria for bioequivalence, test granule formulations cefprozil "Cefprozil for Suspension®" were determined to be bioequivalent whether under fasted or fed conditions by measurement cis-, trans- total cefprozil.
References:
[1] PING PING-LIN, WANG C, PING YAN-LIU, et al. Pharmacokinetics and Bioequivalence of Cefprozil for Suspension and Granule Formulation in Healthy Chinese Volunteers: Two Single-Dose Crossover Studies[J]. Advances in Therapy, 2020, 38: 1130-1142. DOI:10.1007/s12325-020-01593-7.
You may like
Related articles And Qustion
Lastest Price from Cefprozil manufacturers

US $0.00/kg2025-04-22
- CAS:
- 92665-29-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00/KG2025-04-21
- CAS:
- 92665-29-7
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month