Pharmacodynamics of Sulconazole
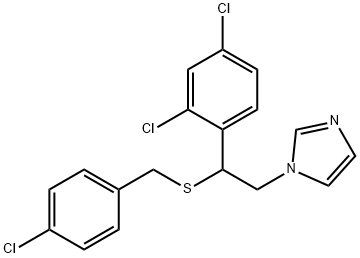
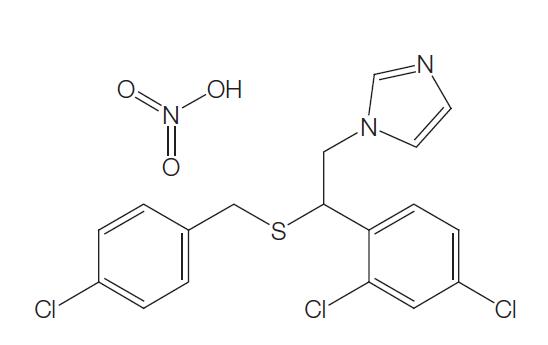
Sulconazole is a substituted imidazole, structurally related to clotrimazole, econazole, and miconazole. It has the chemical name of (7)-1-[2,4-dichloro-b-[(p-chlorobenzyl)thio]phenethyl] imidazole nitrate (C18H15Cl3N2S); the molecular structure is shown below. Sulconazole has a broad spectrum of antifungal activity in vitro and has been shown to be an effective topical antifungal agent for the management of superficial fungal infections of the skin, particularly dermatophytoses and tinea versicolor.
Mechanism of action
The mechanism of action of sulconazole has not been studied well, but it is thought to be similar to the other imidazole derivatives. Inhibition of the sterol 14C-a-demethylation of lanosterol and resultant depletion of ergosterol in the fungal cell membrane is likely to explain part of its antifungal effect. The ensuing increase in cell membrane permeability with release of essential intracellular ions, and interference with RNA and DNA synthesis, have been studied. There is also some evidence for direct cell membrane damage from the fungicidal activity that is dependent on the growth phase, as demonstrated by Beggs.
Pharmacokinetics and Pharmacodynamics
The percutaneous absorption of radiolabeled sulconazole has been examined in healthy male volunteers and animals. In human volunteers, application of 1 g of 3H-sulconazole to intact and abraded skin which was subsequently occluded, resulted in 80% of the dose recovered from the stratum corneum after 8 hours, and 12% of the dose recovered in the feces.
The systemic absorption (9–12% of the dose) was similar for those with intact skin and abraded skin. Percutaneous absorption through shaved skin of animals varied according to species: from 5% to 7% for dogs, 12% to 13% for rats, 6% for guinea-pigs, and 30% to 40% for rabbits. Concentrations of sulconazole achieved in the epidermis and the stratum corneum, and in hair follicle, sebum, or sweat, have not been published. In rats (which have a similar rate of absorption as humans), tissue concentrations of radioactivity 24 hours after a single application of radiolabeled 1% sulconazole cream revealed high concentration in the adrenal glands, and moderate radioactivity in the spleen, brain, blood, and muscle.
Dosage
Sulconazole is available in a number of topical formulations, including a 1% sulconazole nitrate cream and a 1% sulconazole nitrate solution. Superficial dermatomycosis, such as tinea corporis and tinea cruris, should be treated with 1% sulconazole nitrate cream or solution applied twice daily for 3 weeks. For tinea pedis, 1% sulconazole nitrate cream or solution should be applied twice daily for 4 weeks, but for tinea (pityriasis) versicolor, 1% sulconazole nitrate cream or solution should be applied twice daily for 3 weeks. Cutaneous candidiasis can be treated with 1% sulconazole nitrate cream or solution applied twice daily for 3 weeks.
Side effects
Topical sulconazole is well tolerated with a reported local reaction rate of 3.4%. Local reactions tend to occur after 1–2 weeks’ treatment and comprise irritation with pruritus, erythema, and contact dermatitis.