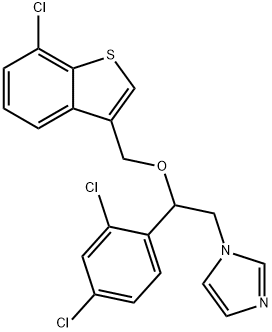
Mechanism of action of Sertaconazole
Sertaconazole nitrate is an imidazole derivative with the chemical name (7)-1-[2,4-dichloro-b-[(7-chlorobenzo[b]thien-3-yl)methoxy] phenethyl]-imidazole nitrate (C20H15Cl3N2OS); the chemical structure is shown below. The substitution of two chlorine atoms in the benzothiophene ring increases the activity of sertaconazole nitrate against Aspergillus, dermatophytes, and Gram-positive bacteria relative to other azoles. It has been developed as a topical antifungal agent for the treatment of dermatologic and vaginal fungal infections.
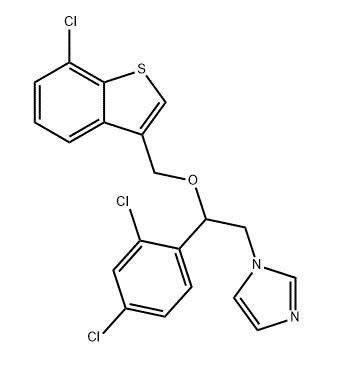
Mechanism of action
Sertaconazole has two primary effects on cell function. First, it inhibits ergosterol synthesis by blockade of the P450-dependent enzyme pathway (lanosterol 14a-demethylase) that catalyzes the methylation of lanosterol to ergosterol, a major constituent of fungal cell membranes. Second, it binds directly to nonsterol lipids in the membrane, which interferes with the regulation of the permeability of fungal cell membranes. Inhibition of sterol synthesis interferes with fungal cell growth, whereas direct interaction with the membrane produces subsequent leakage of intracellular components, particularly adenosine triphosphate (ATP), thereby contributing to immediate cell death. As a result, sertaconazole is an effective fungicidal and fungistatic agent.
Pharmacokinetics and Pharmacodynamics
Sertaconazole contains a benzothiophene group that both augments its antifungal activity and confers a highly lipophilic fragment that enhances the topical action and absorption of the compound into the stratum corneum. Pharmacokinetic studies of sertaconazole nitrate 2% cream performed in healthy volunteers show that the drug is retained in the skin without absorption into plasma after topical application.
Following administration of 100 mg of 2% sertaconazole nitrate to healthy volunteers, concentrations in the stratum corneum were measured at 1409 mg/ml at baseline and 14,550 mg/ml (Cmax) at peak absorption at 24 hours. Similarly, Day (2005) showed that, over a 48-hour exposure period, approximately 38% of the applied dose, or 800 mg, penetrated the skin. These values are considerably higher than the concentrations required to eradicate fungal growth in vitro. Sertaconazole concentrations in the vagina following use of cream, tablet, or ovule appear to be much higher than the MICs obtained in vitro against C. albicans and non-C. albicans species. The analysis of plasma samples after the vaginal application of either cream or tablet (single and repeated doses) did not reveal detectable concentrations of sertaconazole.
Dosage
Sertaconazole is available in a number of formulations, including 2% sertaconazole nitrate cream, 2% gel, 2% solution, 500-mg vaginal tablets, and a 300-mg sustained-release ovule.
Side effects
In clinical trials, cutaneous adverse events occurred in 2% (7/297) of patients receiving sertaconazole 2% cream and in 2% (7/291) of patients receiving placebo. The reported adverse events included contact dermatitis, dry skin, burning skin, application site reaction, and skin tenderness. Epicutaneous tests have shown that sertaconazole does not have a sensitization effect on the skin: 8/202 patients tested with sertaconazole 2% cream and 4/202 patients tested with vehicle exhibited a slight erythematous reaction. Administration of sertaconazole by the vaginal route has been demonstrated as safe. General adverse events were unspecific and a causal relation to the drug was not established in any of the subjects. The local tolerability profile was satisfactory and very few local effects, usually pruritus, burning, and erythema, were reported.
Lastest Price from Sertaconazole nitrate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 99592-32-2
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 500kg/month

US $10.00/KG2025-03-11
- CAS:
- 42461-84-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt