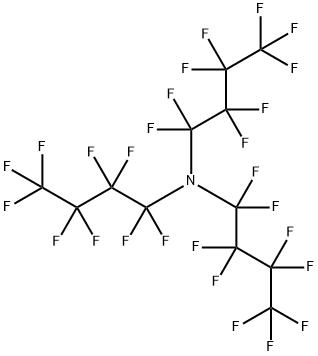
Perfluorotributylamine: Overview, Potential in Tumor Treatment and Toxicity
General Description
Perfluorotributylamine, commonly used in the semiconductor industry for its stability, faces environmental and health concerns due to persistence and bioaccumulation. Its inertness and thermal resistance make it valuable in electronics manufacturing and tumor treatment, where it shows promise in downregulating platelet-derived TGFβ to inhibit metastasis. However, studies on animals have revealed toxic effects, including ocular damage and liver enzyme disruption. Ongoing research is crucial to understand its implications fully. Despite its industrial value, regulatory efforts are necessary to address potential drawbacks and explore alternative compounds with lower environmental impact.

Figure 1. Perfluorotributylamine
Greenhouse Gas
Perfluorinated compounds impact the Earth’s radiative balance. Perfluorotributylamine (PFTBA) belongs to the perfluoroalkyl amine class of compounds; these have not yet been investigated as long-lived greenhouse gases (LLGHGs).
Atmospheric measurements of PFTBA made in Toronto, ON, detected a mixing ratio of 0.18 parts per trillion by volume. An instantaneous radiative efficiency of 0.86W m-2 ppb-1 was calculated from its IR absorption spectra, and a lower limit of 500 years was estimated for its atmospheric lifetime.
PFTBA has the highest radiative efficiency of any compound detected in the atmosphere. If the concentration in Toronto is representative of the change in global background concentration since the preindustrial period, then the radiative forcing of PFTBA is 1.5 × 10-4 W m-2. Researchers calculate the global warming potential of PFTBA over a 100 year time horizon to be 7100. Detection of PFTBA demonstrates that perfluoroalkyl amines are a class of LLGHGs worthy of future study.1
Potential in tumor treatment
Tumor metastasis contributes to the low overall survival of tumor patients, while transforming growth factor-β (TGFβ) has been recognized as a prominently promoting factor in the development of tumor metastasis. Platelets reserve abundant TGFβ, which will be secreted to peripheral blood after activation, and they are the dominant source of circulating TGFβ. Therefore, downregulation of platelet-derived TGFβ is expected to inhibit the metastasis of circulating tumor cells. Unfolded human serum albumin (HSA)-coated perfluorotributylamine (PFTBA) nanoparticles were constructed by Xiaozhi Zhao et.al., to display a favorable platelet delivery and an antiplatelet effect to downregulate platelet-derived TGFβ in vitro and in blood plasma. PFTBA@HSA-mediated TGFβ downregulation impaired epithelial-mesenchymal transition of tumor cells as well as their migration and invasion behaviors and enhanced immune surveillance of NK cells. Intravenous injection of PFTBA@HSA effectively reduced tumor metastasis on the lungs or liver to improve the survival rate of mice on multiple metastatic models, including CT26 colon cancer, B16F10 melanoma, and 4T1 breast cancer. Compared with the clinical antiplatelet drug ticagrelor, PFTBA@HSA reduced bleeding risk when displaying a favorable downregulation on platelet-derived TGFβ, thereby obtaining a higher therapy benefit. Together, this study confirmed that downregulation of platelet-derived TGFβ by PFTBA@HSA will be a potential approach and therapeutic candidate for the prevention of tumor metastasis.2
Toxicity
Although PFTBA hardly contributes to the formation of photochemical oxidants in the troposphere, it could be initiated by reaction with reactive ions (e.g., O+, O2+, O2−, and H3O+) under thermal conditions such as pyrolysis, lightning, and plasma. This is similar to the thermal decomposition of perfluorocompound (e.g., NF3) in the plasma environment. On the other hand, some toxic products such as hydrogen fluoride (HF), fluorine (F2), and carbonyl fluoride (COF2) may be generated from the combustion of mostly fluorinated compounds.
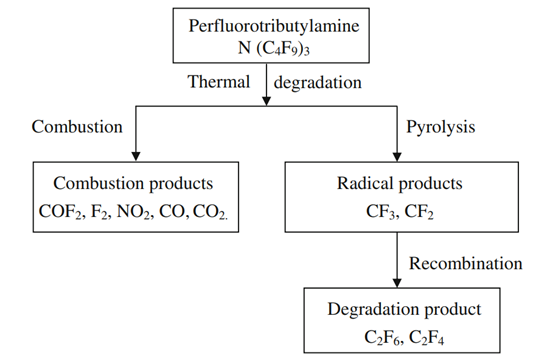
Figure 2. Probable thermal degradation mechanism of perfluorotributylamine.
Being analogous to the initial breakdown of perfluorocarbons that occurred at the susceptible bond (C–C), thermal degradation of PFTBA would proceed to generate the radicals (i.e., CF3 and CF2). As a result, PFTBA will decompose into tetrafluoroethylene (C2F4) and hexafluoroethane (C2F6) under the pyrolysis by spark and lightning. This situation is slightly similar to toxic product (i.e., tetrafluoroethylene C2F4, hexafluoropropylene C3F6, and perfluoroisobutylene C4F8) formation from perfluorocarbon pyrolysis processes at high temperature. In summary, Fig. 2 shows the probable thermal degradation products of perfluorotributylamine (PFTBA) by the process of combustion and pyrolysis.3
potent greenhouse gas. DOI 10.1007/s11027-015-9684-6References:
[1] ANGELA C. HONG. Perfluorotributylamine: A novel long-lived greenhouse gas[J]. Geophysical Research Letters, 2013, 40 22: 5827-6015. DOI:10.1002/2013GL058010.[2] LIFENG LUO, JINHUI WU*. Perfluorotributylamine-Loaded Albumin Nanoparticles Downregulate Platelet-Derived TGFβ to Inhibit Tumor Metastasis[J]. ACS Nano, 2023, 17 16: 15217-16286. DOI:10.1021/acsnano.3c00295.
[3] TSAI W. Environmental implications of perfluorotributylamine—a potent greenhouse gas[J]. Mitigation and Adaptation Strategies for Global Change, 2017, 22 1: 225-231. DOI:10.1007/s11027-015-9684-6.
Related articles And Qustion
Lastest Price from Perfluorotributylamine manufacturers
US $0.00-0.00/kg2025-11-03
- CAS:
- 311-89-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1ton

US $0.00-0.00/KG2025-08-07
- CAS:
- 311-89-7
- Min. Order:
- 1KG
- Purity:
- 0.98 min
- Supply Ability:
- 1 tons/year