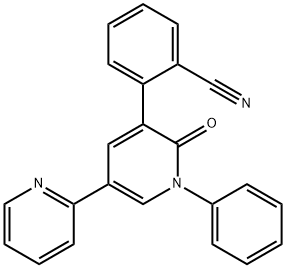
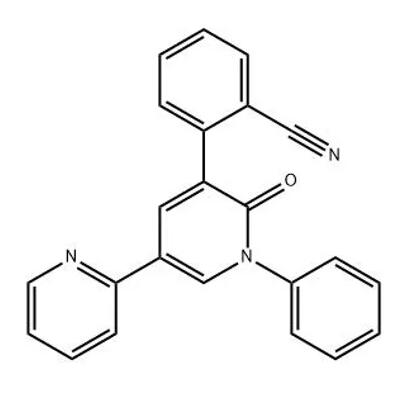
Perampanel: A Promising AMPA Receptor Antagonist for Epilepsy Treatment
Description
Perampanel is used alone or together with other medicines to treat certain types of epilepsy, such as partial onset seizures and generalized tonic-clonic seizures. It works in the brain to prevent seizures. Perampanel will not cure epilepsy and will only control the seizures as long as you continue to take it.
Preclinical Studies
AMPA receptors play a key role in mediating the action of glutamate at the excitatory synapse. Preclinical research showed the AMPA receptor blockade to constitute a promising target for antiepileptic drug therapy. In animal models, perampanel proved to be protective against seizures and reduce seizure severity and duration. Four phase-3 randomized controlled trials (3 in patients with focal seizures and one in primary generalized tonic-clonic seizures in idiopathic generalized epilepsy) have been completed. In focal (partial) onset seizures, perampanel (4, 8, and 12 mg) significantly reduced seizure frequency per 28 days (23.3%-28.8% vs 12.8%; P < .01) and responder rates (≥50% reduction in seizures) (28.5%-35.3% vs 19.3%; P < .05) compared with placebo. In primary generalized tonic-clonic seizures, perampanel 8 mg resulted in greater reduction in seizure frequency per 28 days (-76.5% vs -38.4%; P < .0001) and responder rate (64.2% vs 39.5%; P = .0019) than placebo.1
Clinical Practice
Focal seizures
Perampanel has been approved as adjunctive therapy for focal seizures with or without secondary generalization based on three phase-3 multicenter randomized controlled trials (RCTs) and an open-label extension study. Pooled analysis of 1478 patients with refractory epilepsy in the phase-3 RCTs showed that median changes in focal seizure frequency per 28 days and responder rates were significantly greater with perampanel at 4, 8, and 12 mg than with placebo. Median changes in complex focal plus secondarily generalized seizure frequency were also significantly greater with perampanel. The most common treatment-emergent adverse events (TEAEs) included dizziness, somnolence, headache, fatigue, and others, with most being mild to moderate in severity. Psychiatric TEAEs such as sleep disorder, anxiety, aggression, and confusional state were reported, with hostility/aggression events occurring more frequently with perampanel, although serious psychiatric AEs were uncommon. Long-term data from an open-label extension study demonstrated a median percent seizure reduction of 70.6% and a responder rate of 67.9% after 4 years of treatment with perampanel, with no new safety signals identified. 2
References:
[1] HEIDRUN POTSCHKA E T. Perampanel: Does it have broad-spectrum potential?[J]. Epilepsia, 2018, 60 S1: 1-67. DOI:10.1111/epi.14456.[2] BERNHARD J. STEINHOFF. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: A pooled analysis of three phase III studies[J]. Epilepsia, 2013, 54 8: 1335-1503, e99-e116, 1505-1511. DOI:10.1111/epi.12212.
Related articles And Qustion
See also
Lastest Price from PeraMpanel manufacturers
US $0.00/kg2024-03-28
- CAS:
- 380917-97-5
- Min. Order:
- 25kg
- Purity:
- 98%-99%
- Supply Ability:
- Inquiry
US $0.00-0.00/kg2023-04-11
- CAS:
- 380917-97-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg