Diethyltoluenediamine: Uptake, Distribution, and Enhanced Properties in Polyurethaneurea and Epoxy Resins
General Description
Diethyltoluenediamine (DETDA) is a cycloethylated analog of toluenediamine (TDA) and, like TDA, consists of 2,4 and 2,6-diamine isomers. DETDA is used as a replacement for TDA in certain applications. Studies have shown that the major elimination route for DETDA is the urinary system, with most markers excreted within the first 24 hours. Urinary excretion of 2,6-DETDA is higher than that of the 2,4-isomer. Both 2,4- and 2,6-DETDA covalently bind to rat liver DNA and liver protein.

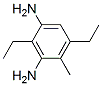
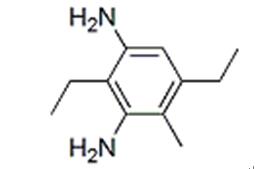
Figure 1. Diethyltoluenediamine
Applications
Effects in curing epoxy resins
Mixtures of DETDA and polyoxypropyleneamines (Jeffamine® D-230 and D-400) can be used to cure epoxy resins. Mixtures of Jeffamine® and DETDA produce cured epoxy systems with higher impact strength than DETDA alone. Furthermore, the heat distortion temperature, modulus, and hardness of epoxies cured with such blends were much higher than those cured with Jeffamine® alone. Furthermore, the bond strength was maximized at intermediate levels of curing agent mix ratios.
Healing Agents
Using bisphenol A epoxy and diglycidyl ether of DETDA, the cured epoxy network was converted to a repairable system using a phenoxy resin and a low molecular weight poly(bisphenol A-co-epichlorohydrin) thermoplastic modifier. Using a functionally terminated low molecular weight poly(bisphenol A-co-epichlorohydrin) thermoplastic modifier as a healing agent, it was shown that salicylic acid or neutralized sodium salicylate groups produced healing effects similar to high molecular weight non-functional phenoxy resins as measured by single-ended notched beam testing. The miscibility of the two thermoplastics in the bisphenol A/diethyltoluenediamine diglycidyl ether system was evaluated using differential scanning calorimetry and dynamic mechanical thermal analysis and determined to be important in promoting healing. Near-infrared spectroscopy showed that the network structure was not affected by thermoplastic modification, suggesting that healing occurs primarily through physical or noncovalent mechanisms rather than covalent bonds.
Chain Extenders
The physical properties of aqueous polyurethaneurea (PUU) dispersions prepared with DETDA were found to be similar to or better than those of aqueous PUU dispersions prepared with EDA. Water resistance and mechanical properties of aqueous PUU films extended with DETDA were significantly improved compared to those of aqueous PUU films extended with EDA; these improvements were attributed to the strong hydrogen bonds in the urea carbonyl groups and the ordered structure of the hard segments in the system. Among all the PUU films studied, the DETDA-extended PUU film with a hard segment content of 40 wt.% and a DMPA unit content of 4.0 wt.% had the lowest water uptake (2.6 wt.%). It was observed that the hydrophobic surface of DETDA-extended PUU films modified with a small amount of aminoethylaminopropylpolydimethylsiloxane (AEAPS) was enhanced and its hydrophobicity was enhanced with further increase in AEAPS content.
Reference
1. BABIN M C. The Uptake and Distribution of Diethyltoluenediamine in the Male Sprague Dawley Rat. 1900.
2. Lin J, Chun PH, Yong LC. Polyurethaneurea aqueous dispersions prepared with diethyltoluenediamine as chain extender. Journal of Coatings Technology and Research. 2007. 4(1): 59-66.
3. Huang JC, Ying TC. Blend-Curing of Epoxies with Jeffamine? and Diethyltoluenediamine. Journal of Polymer Engineering. 1996. 16(1): 51-72.
4. R. VARLEY. Low-molecular-weight thermoplastic modifiers as effective healing agents in mendable epoxy networks[J]. Journal of Intelligent Material Systems and Structures, 2014, 28 1: 107-117. DOI:10.1177/1045389X13507348.
Related articles And Qustion
Lastest Price from Diethyltoluenediamine manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 68479-98-1
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-05-07
- CAS:
- 68479-98-1
- Min. Order:
- 1KG
- Purity:
- 99% HPLC
- Supply Ability:
- 1KG 100KG 1MT