Diethyltoluenediamine: properties, applications of in polymer and safety
General Description
Diethyltoluenediamine is a diamine compound with two aromatic toluene rings connected to two amino groups. It has unique physical and chemical properties, including low volatility, solubility in water and polar solvents, and stability under normal conditions. It finds applications in the polymer industry as a chain extender or crosslinking agent in polyurethane, epoxy, and polyurea systems, enhancing mechanical strength, chemical resistance, and overall performance. However, it should be handled with caution due to its potential irritant and sensitizing effects, as well as its suspected carcinogenic properties.

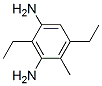
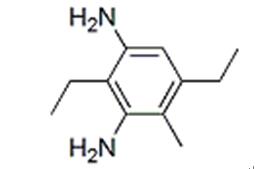
Figure 1. Diethyltoluenediamine
Properties
Diethyltoluenediamine is a diamine derivative that shares structural similarities with diethylenetriamine, a widely used diamine drug. Diethyltoluenediamine possesses distinct physical and chemical properties due to its two aromatic toluene rings connected to two amino groups. Diethyltoluenediamine consists of two aromatic toluene rings connected to two amino groups. Its molecular formula is C11H18N2, and it has a molecular weight of 178.27 g/mol. It appears as a colorless to yellowish liquid with a characteristic amine odor. It has a relatively low vapor pressure and a higher boiling point compared to other diamines, which gives it lower volatility at normal temperatures. It is soluble in water, as well as in polar solvents like alcohol and dimethylformamide. This property makes it easily dispersible in various solvent-based systems. Due to the presence of amino groups, It exhibits reactivity similar to other diamines. It demonstrates stability under normal conditions. However, its reactivity may increase in the presence of certain catalysts or elevated temperatures, leading to polymerization or degradation of the compound. Diethyltoluenediamine can form salts with acids, indicating acidic properties. These salt formations are commonly utilized in chemical processes and synthesis. 1
Applications in polymer
Diethyltoluenediamine finds various applications in the polymer industry. Its unique properties make it a valuable component in the formulation of polymers, coatings, adhesives, and elastomers. It is widely used as a chain extender or crosslinking agent in the production of polyurethane polymers. It reacts with isocyanate groups to form a urea linkage, contributing to the formation of highly durable and flexible polyurethane products. DETDA helps improve the mechanical strength, chemical resistance, and overall performance of polyurethane coatings, adhesives, and elastomers. It acts as a curing agent for epoxy resins, promoting the crosslinking reaction between epoxy functionalities. This results in the formation of tough and durable thermosetting epoxy polymers. These cured epoxy systems exhibit excellent adhesion properties, chemical resistance, and thermal stability, making them suitable for applications such as coatings, composites, and electrical encapsulation. It is employed as a key component in polyurea coating systems, primarily acting as an amine chain extender. When reacted with isocyanates, it participates in the rapid crosslinking process, leading to the formation of robust and abrasion-resistant polyurea coatings. These coatings are extensively used in industries requiring corrosion protection, such as automotive, infrastructure, and marine applications. In summary, diethyltoluenediamine plays a critical role in the polymer industry. It is employed in the production of polyurethanes, epoxy resins, polyurea coatings, adhesives, and elastomers. Diethyltoluenediamine enhances the mechanical strength, chemical resistance, adhesion, and overall performance of various polymer-based products across numerous industrial applications. 2
Safety
Diethyltoluenediamine has low acute toxicity, and inhalation of its fumes or contact with the skin or eyes can cause irritation and corrosion. It is considered a sensitizing agent and may cause skin sensitization in some individuals. Diethyltoluenediamine is a suspected carcinogen, and long-term exposure to high levels of the compound has been associated with an increased risk of liver cancer. It is also suspected of causing other types of cancer, including bladder cancer and non-Hodgkin lymphoma. Diethyltoluenediamine is flammable in its pure form, but it is not considered a severe fire hazard. It is also irritating to the respiratory system and may cause irritation to the nose, throat, and lungs when inhaled. Overall, diethyltoluenediamine should be handled with caution and handled in a well-ventilated area to minimize exposure. Contact with skin or eyes should be avoided, and individuals who handle the compound should wear appropriate personal protective equipment. 3
Reference
1. PubChem. COMPOUND SUMMARY: 1,3-Benzenediamine, 2,4-diethyl-6-methyl. National Library of Medicine, 2005, CID:74980.
2. Jiang L, Hu CCP. Polyurethaneurea aqueous dispersions prepared with diethyltoluenediamine as chain extender.Journal of Coatings Technology&Research, 2007.
3. SAFETY DATA SHEETS: Diethyltoluenediamine. LookChem, 2017.
Related articles And Qustion
Lastest Price from Diethyltoluenediamine manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 68479-98-1
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-05-07
- CAS:
- 68479-98-1
- Min. Order:
- 1KG
- Purity:
- 99% HPLC
- Supply Ability:
- 1KG 100KG 1MT