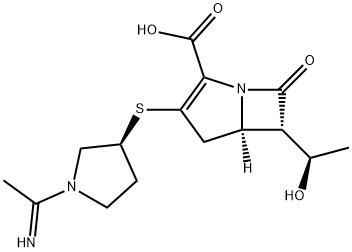
Panipenem: Antimicrobial Activity, Susceptibility, Toxicity and Clinical Uses
Panipenem is a parenteral carbapenem with a broad spectrum of in vitro activity against both Gram-positive and Gram-negative bacteria (Miyadera et al., 1991; Shimada, 1994; Goa and Noble, 2003). As it is not stable to hydrolysis by renal dehydropeptidase-I (DHP-1) (Hikida et al., 1992), panipenem requires concomitant administration of a DHP-1 inhibitor, such as betamipron. Betamipron is an organic anion tubular transport inhibitor with very low toxicity that inhibits the active transport of panipenem in the renal cortex (Hirouchi et al., 1994; Enomoto et al., 2002).
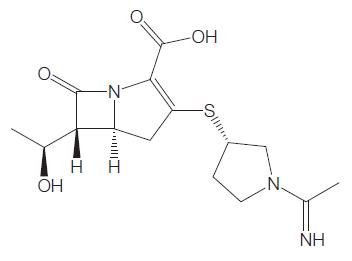
The molecular formula for biapenem is C15H21N3O4S and its molecular weight is 339.41; the chemical structure is shown in Figure 42.1.
Biapenem is not currently licensed for use, and its role compared with other available carbapenems, such as imipenem and meropenem is currently unclear.
ANTIMICROBIAL ACTIVITY
There are no susceptibility standards for panipenem currently published by the Clinical Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST). The standards for imipenem and meropenem are used for determination of susceptibility. Most of the information has been obtained from Japanese studies.
Gram-positive bacteria
Panipenem demonstrates good antimicrobial activity against Grampositive aerobic bacteria (Table 42.1) (Watanabe et al., 1999; Mikamo et al., 2000; Fujimura et al., 2006). However, methicillin-resistant Staphylococcus aureus, methicillin-resistant S. epidermidis, and Enterococcus spp. show intermediate to full resistance when the imipenem breakpoints are used. The activity is similar to that of imipenem and is 2- to 4-fold better than that of meropenem. The in vitro efficacy of panipenem against Gram-positive pathogens is summarized in Table 42.1.
Gram-negative aerobic bacteria
Most of the Enterobacteriaceae, including Escherichia coli, Klebsiella spp., Proteus spp., Providencia spp., Morganella morganii, Citrobacter spp., Enterobacter spp., and Serratia marcescens, are susceptible to panipenem (see Table 42.2) (Yoshida et al., 2006), together with extended-spectrum beta-lactamase (ESBL)-producing E. coli and K. pneumoniae (Nakamura and Komatsu, 2005).
Anaerobic bacteria
Panipenem demonstrates good antimicrobial activity against anaerobic bacteria (see Table 42.3) (Watanabe et al., 1991; Mikamo et al., 2000; Fujimura et al., 2006). Some in the Bacteroides fragilis group, and Clostridium difficile show MIC Z8 mg/ml (Fujimura et al., 2006).
MODE OF DRUG ADMINISTRATION AND DOSAGE
Panipenem is only available for parenteral administration (Miyadera et al., 1991).
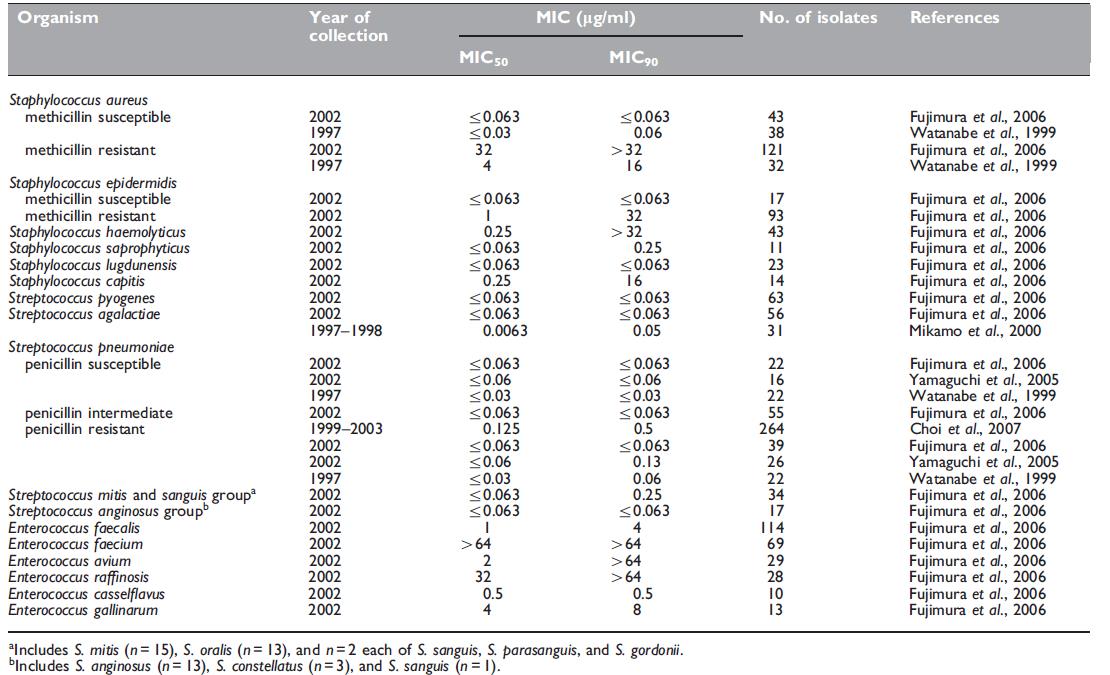
Table 42.1 Minimum inhibitory concentrations (MICs, mg/ml) of panipenem for Gram-positive isolates.
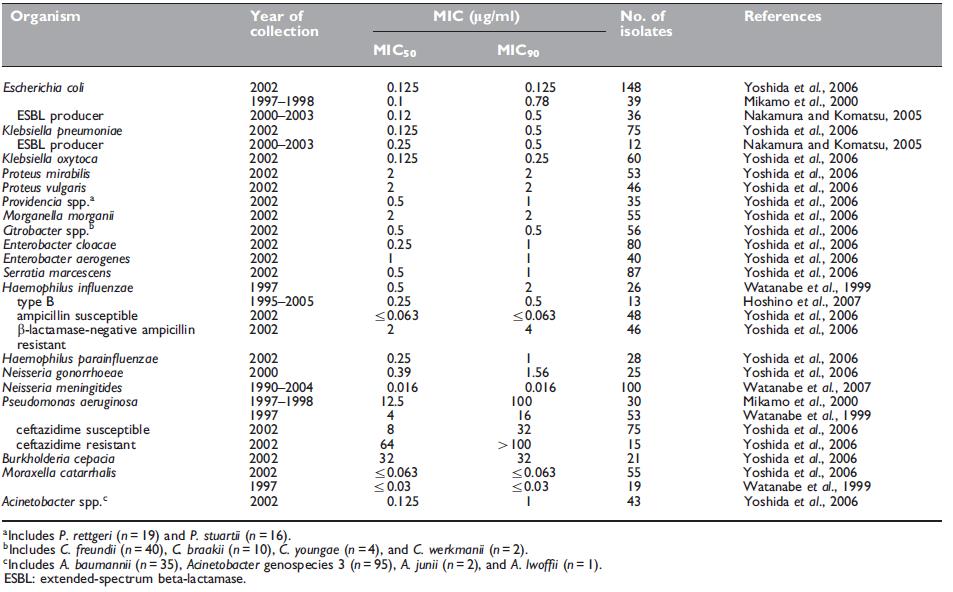
Table 42.2 Minimum inhibitory concentrations (MICs, mg/ml) of panipenem for Gram-negative isolates.
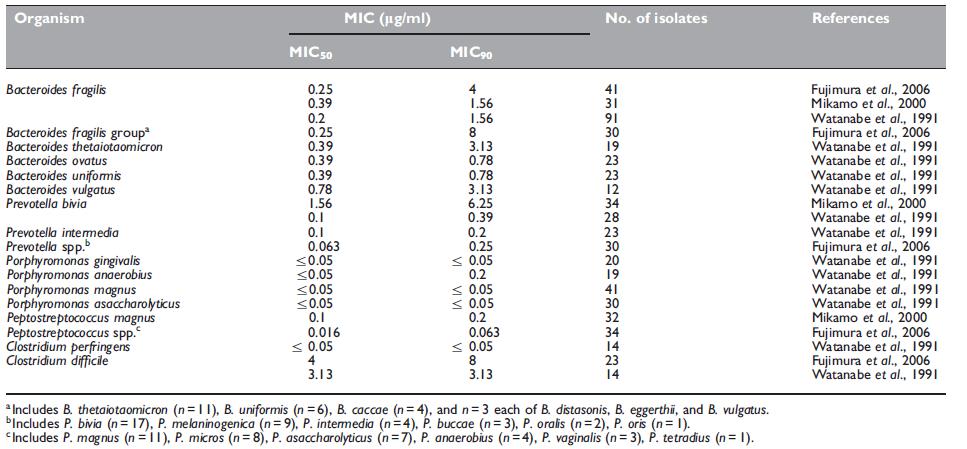
Table 42.3 Minimum inhibitory concentrations (MICs, mg/ml) of panipenem for anaerobic strains.
a. Adults
In Japan, the recommended routine dose for adults of panipenem is 500 mg every 12 hours, but 1.0 g every 12 hours is used if the patient is in a critical or refractory condition (Shimada, 1994).
b. Newborn infants and children
In Japan, a dose of 10–30 mg/kg every 8 hours is recommended for children (Shimada, 1994).
TOXICITY
When panipenem was administered to 2231 adult patients and 380 pediatric patients, adverse events occurred in 68 (3.0%) adult patients and nine (2.4%) pediatric patients. Most of the adverse events were gastrointestinal symptoms, such as diarrhea, nausea, and vomiting (48 cases), and allergic symptoms, such as rash and urticaria (38 cases) (Shimada, 1994).
CLINICAL USES OF THE DRUG
The efficacy of panipenem has been investigated in numerous studies in Japan. Most of the studies were small and noncomparative in design.
As noted above under 4. Mode of drug administration and dosage, the routine dose of panipenem used for adults in Japan is 500 mg intravenously every 12 hours, with 1.0 g every 12 hours reserved for patients who are critically ill; the pediatric dose is 10–30 mg/kg every 8 hours (Shimada, 1994). Based on data from the 2089 patients who underwent clinical trials in Japan (1750 adults, 339 children), panipenem proved effective in 82.0% of adult cases and 96.2% of pediatric cases (Shimada, 1994).
References
Aoki N, Usuda Y, Koda Y et al. (1991). Clinical pharmacology and efficacy of
panipenem/beramipron. Chemotherapy 39 (Suppl. 3): 372 (in Japanese).
Arata J, Akiyama H, Kanzaki H et al. (1992). A multicenter study on
panipenem/betamipron in dermatology. Jpn J Antibiot 45: 197 (in Japanese).
Choi SH, Park SJ, Jun JC et al. (2007). Comparative in vitro activities of
carbapenem antimicrobial agents against 264 penicillin-resistant
Streptococcus pneumonia isolates from Korea. Diagn Microbiol Infect Dis 58:
141.
Enomoto A, Takeda M, Shimoda M et al. (2002). Interaction of human organic
anion transporters 2 and 4 with organic anion transport inhibitors. J
Pharmacol Exp Ther 301: 797.
Fujii R, Abe T, Tajima Tet al. (1992). Overall clinical evaluation of panipenem/
betamipron against infections in the pediatric field. Jpn J Antibiot 45: 208 (in
Japanese).
Fujimura T, Yoshida I, Jinushi Y (2006). Antimicrobial susceptibility of clinical
isolates of aerobic gram-positive cocci and anaerobic bacteria in 2002. Jpn J
Chemother 54: 330 (in Japanese).
Goa KL, Noble S (2003). Panipenem/betamipron. Drugs 63: 913.
Goto Y, Ichimiya T, Ikuta M et al. (1991). In vitro antibacterial activity of
panipenem/betamipron, a carbapenem antibiotic, and its clinical study
in respiratory airway infections. Chemotherapy 39 (Suppl. 3): 441 (in
Japanese).
Hikida M, Kawashima K, Yoshida M, Mitsuhashi S (1992). Inactivation of new
carbapenem antibiotics by dehydropeptidase-I from porcine and human
renal cortex. J Antimicrob Chemother 30: 129.
Hirouchi Y, Naganuma H, Kawahara Y et al. (1994). Preventive effect of
betamipron on nephrotoxicity and uptake of carbapenems in rabbit renal
cortex. Jpn J Pharmacol 66: 1.
Morimoto K, Kinoshita H, Nakatani S et al. (1991). Panipenem/betamipron in
the treatment of patients with surgical infections. Chemotherapy 39 (Suppl.
3): 572 (in Japanese).
Nakamura T, Komatsu M (2005). Susceptibility of ESBL-producing Escherichia
coli and Klebsiella pneumonia to various antibacterial agents. Jpn J Antibiot 58:
1 (in Japanese).
Nakashima M, Uematsu T, Kanamaru M et al. (1991a). Phase I study of
panipenem/betamipron I – single dose study. Chemotherapy 39 (Suppl. 3):
242 (in Japanese).
See also
Lastest Price from Panipenem manufacturers

US $1.00-1.00/KG2025-01-03
- CAS:
- 87726-17-8
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 50tons