p-Tolualdehyde: Applications in Analytical Chemistry and its Production Method
Introduction
p-Tolualdehyde is a crucial compound in analytical chemistry, aiding in the quantification of hydrazine and acetylhydrazine in biological samples through derivatization for improved detection using HPLC-MS/MS. Its use enhances separation and detection on a C18 column. The innovative production of p-Tolualdehyde from p-xylene using a photoinduced electron transfer method exhibits 100% high selectivity, avoiding byproducts. Besides, a related method generates p-Tolualdehyde and hydrogen peroxide simultaneously, showcasing dual production benefits for economic value and practicality.

Figure 1. p-Tolualdehyde
Applications in Analytical Chemistry
In this study, a new derivatization reagent, p-tolualdehyde, was selected to simultaneously derivatize hydrazine and acetylhydrazine1. We speculated that hydrazine and acetylhydrazine were derivatized to improve their molecular polarities and mass response due to the incorporation of phenyl group for the suitable analysis by HPLC–MS/MS. Result shows that chromatographic retention and mass spectral response signals were improved.
The hydrazine group of hydrazine and acetylhydrazine reacted with aldehyde group of p-tolualdehyde yielded a single hydrazone product through condensation, as shown in the figure below.
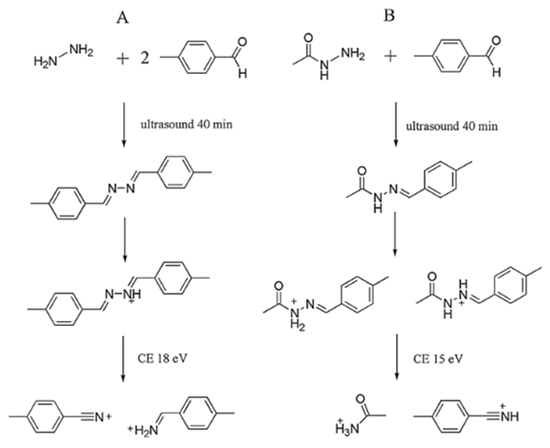
Figure 2. Derivatization of hydrazine (A) and acetylhydrazine (B) (mass ratio of A:B = 1:10) with p-tolualdehyde.
After derivatization, the predominant characteristic fragmentations of the two derivatization products were obtained. The fragment ions of derivatized hydrazine (m/z 237.1) were m/z 119.9, 117.9 and 90.9. And the fragment ions of derivatized acetylhydrazine (m/z 176.9) were m/z 117.8, 134.9 and 90.8. Their daughter ions were stable and the intensities were high, which were helpful for the following identification and quantification analysis. In addition, the derivatization products were also confirmed by quadrupole-time of flight mass spectrometry (Q-TOF MS). The accurate m/z of hydrazine and acetylhydrazine derivatization products were m/z 237.1394 and 177.1033, respectively. Compared with theoretical values, both the mass errors were less than 3 ppm.
Production Method
p-xylene to p-tolualdehyde
The 100% selective oxygenation of p-xylene to p-tolualdehyde is initiated by photoinduced electron transfer from p-xylene to the singlet excited state of 10-methyl-9-phenylacridinium ion under visible light irradiation, yielding p-tolualdehyde exclusively as the final oxygenated product.2
Mechanism
The reason for the high selectivity in the photocatalytic oxygenation of p-xylene is discussed on the basis of the photoinduced electron transfer mechanism.
The 100% selective photocatalytic oxygenation of p-xylene is made possible by the difference in the reactivity of p-xylene and the oxygenated product, p-tolualdehyde. The fluorescence lifetimes (τ) of AcrH+ (λem ) 488 nm) in the absence and presence of xylenes, toluene, or the corresponding aldehydes were determined by using a time-resolved fluorescence spectrofluorophotometer. The rate constants of fluorescence quenching kq ( Kqτ-1) by photoinduced electron transfer are determined from the slopes of the linear Stern-Volmer plots of τ0/τ (τ0 ) 37 ns in MeCN 13) vs the quencher concentration.2
References:
[1] LU SONG . Simultaneous quantitation of hydrazine and acetylhydrazine in human plasma by high performance liquid chromatography-tandem mass spectrometry after derivatization with p-tolualdehyde[J]. Journal of Chromatography B, 2017, 1063: 1-258. DOI:10.1016/j.jchromb.2017.08.036.[2] KEI OHKUBO S F. 100 Selective Oxygenation of p-Xylene to p-Tolualdehyde via Photoinduced Electron Transfer[J]. Organic Letters, 2000, 2 23: 3531-3755. DOI:10.1021/ol0065571.
You may like
See also
Lastest Price from p-Tolualdehyde manufacturers

US $55.00/kg2025-04-21
- CAS:
- 104-87-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons
US $0.00-0.00/KG2025-04-04
- CAS:
- 104-87-0
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton