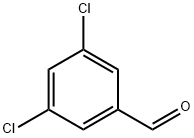
3,5-Dichlorobenzaldehyde - reaction / application on synthetic works
3,5-Dichlorobenzaldehyde is an important organic intermediate to synthetize substituted benzene products.
The following example is about its application on the synthesis of 4H-pyrans derivatives [1]
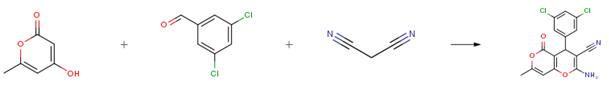
The aldehyde (1.0 mmol), malononitrile (1.0 mmol), active methylene compounds (1.0 mmol) [hydroxy-6-methyl-2-pyrone, 4-hydroxycoumarin or dimedone], TMDPS (5mol%) were ground vigorously using the planetary ball mill at room temperature for 30 min. The progress of the reaction was monitored by TLC. On completion of the reaction, the product was extracted by hot ethyl acetate which after evaporation of the solvent, the solid crude product was purified just by recrystallization from ethanol without tedious column chromatographic purification. The TMDPS was concentrated under reduced pressure, and the reaction was recharged with new reactants for another reaction. The structure of each purified 4H-pyran-annulated heterocyclic scaffolds was confirmed by melting point and spectral studies including FT-IR, 1H NMR, 13C NMR, and elemental analysis.
The following example is about its application on the synthesis of biscoumarin derivatives [2]

A mixture of 3,4,5-trifluorobenzaldehyde (3,5-dichlorobenzaldehyde, 4-methyl thiobenzaldehyde or2,4-dihydroxybenzaldehyde) (10 mmol) and 4-hydroxycoumarin (20 mmol) was dissolved in 100 mL of EtOH. A few drops of piperidine were added, and the mixture was stirred for 3 h at room temperature. After reaction completion as determined by TLC, water was added until precipitation occurred. After filtering the precipitates, they were sequentially washed with ice-cooled water and ethanol and then dried in a vacuum. 3,3′-(3,4,5-Trifluorobenzylidene)-bis-(4-hydroxycoumarin) (1): Yield: 57%. 230-231 °C.
The following example is about its application on the synthesis of bis(naphthalen-2-yl-sulfane) derivatives [3]

A mixture of 2-thionaphthol (2 mmol), aromatic aldehyde (1 mmol) and PVPP-BF3 (0.05 g) was stirred at reflux condition in the 1,2-dichloroethan for the appropriate time. Completion of the reaction was indicated by TLC monitoring. The reaction mixture was cooled to ambient temperature, and the PVPP-BF3 was filtered off. The filtrate was concentrated to dryness, and the crude solid residue was recrystallized from ethanol to afford pure crystals of the proper bis(naphthalen-2-yl-sulfane) derivatives in 86-98 percent yields. The products were characterized by FT-IR, 1H NMR, 13C NMR, electron ionization mass spectrometry and physical constants. 2.4.5. (3,5-dichlorophenylmethylene)bis(naphthalen-2-yl-sulfane). m.p. 125-128 °C. IR (KBr): (νmax, cm-1) 3049, 2926, 1569, 1496, 1384, 1344, 1190, 1118, 952, 896, 858, 808, 738. 1H NMR (CDCl3): δ = 5.50 (s, 1H), 7.22-7.84 (m, 17H) ppm. 13C NMR δ = 59.8, 126.5, 126.7, 126.8, 127.6, 127.7, 128.2, 128.7, 129.8, 130.7, 132.5, 132.8, 133.5, 134, 135, 143. EI-S (m/z): 477 (M + H).
References
1. Khaligh NG, Mihankhah TJ, Mohd R. Synthesis of new low-viscous sulfonic acid-functionalized ionic liquid and its application as a Brönsted liquid acid catalyst for the one-pot mechano synthesis of 4H-pyrans through the ball milling process[J]. Journal of Molecular Liquids, 2019, 277:794 – 804.
2. Sui YP, Huo HR, Xin JJ, Li J, Li XJ, Du XL, Ma H, Li MK, McPhee DJ. Antibacterial and antitumor activities of biscoumarin and dihydropyran derivatives[J]. Molecules, 2015, 20(9):17614-17626.
3. Mokhtary M, Refahati S. Polyvinylpolypyrrolidone-supported boron trifluoride (PVPP-BF3): Mild and efficient catalyst for the synthesis of 14-aryl-14H-dibenzo [a,j] xanthenes and bis(naphthalen-2-yl-sulfane) derivatives[J]. Dyes and Pigments, 2013, 99(2):378-381.
You may like
See also
Lastest Price from 3,5-Dichlorobenzaldehyde manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 10203-08-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt

US $0.00-0.00/KG2025-01-03
- CAS:
- 10203-08-4
- Min. Order:
- 10mg
- Purity:
- 99%HPLC
- Supply Ability:
- 2000tons