Overview of bf3 polarity
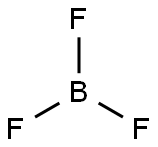
Boron trifluoride (BF3) is composed of a boron (B) atom bonded by three fluoride (F) atoms through single covalent bonds.
Non-Polar bf3
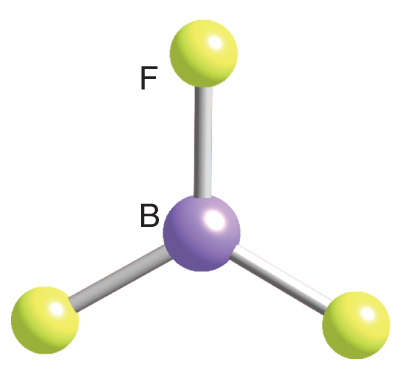
bf3(Boron Trifluoride) is Non-Polar because of its highly symmetric shape. It has a Trigonal Planar geometry which cancels out the dipole moments of the three BF bonds making the resultant Dipole Moment of the compound equal to 0 (Zero).
Molecular Structure
The molecular geometry of BF3 is a trigonal planer with three peripheral atoms surrounding one central atom in a single plane. The bond lengths of the three B-F bonds are similar, and all the bonds are separated by 120°. The molecular structure of BF3 is such that the bonds combine to form an equilateral triangle, making BF3 a highly symmetrical molecule.
Character
Typically this compound is highly reactive, being able to corrode metals including stainless steel. It is utilized as a catalyst in a wide variety of organic chemistry reactions.
BF3 typically behaves as an acid since the presence of fluorines produces an "electron-deficient" structure. This character is evident in many of the different organic reactions which utilize BF3.