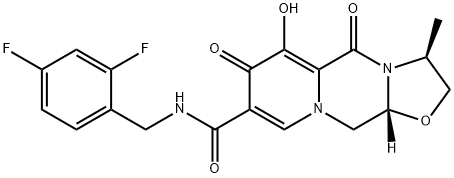
How is GSK744 synthesised?
Synthesis of GSK744 (Cabotegravir)
GSK744 (Cabotegravir) is synthesised using Cabotegravir Pyridinone as a raw material through a series of chemical reactions. The specific synthetic pathway is as follows:
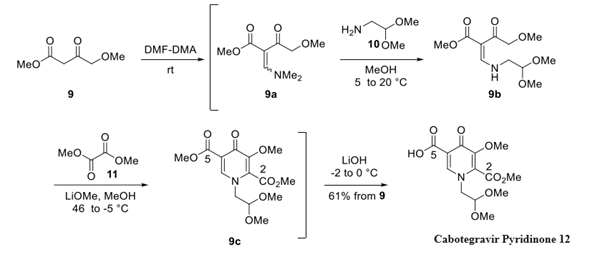
Step 1: Preparation of Cabotegravir Pyridinone
Starting from 4- methoxyacetoacetate 9, condensation with DMF-DMA at room temperature generated intermediate vinylogous amide 9a, which was not purified. The concentration of the reaction mixture to remove excess reagent and the subsequent addition of dimethyl acetal 10 in MeOH generated intermediate 9b.
The concentration and treatment of the resulting crude product with dimethyl oxalate 11 and lithium methoxide in warm MeOH triggered a cyclization to secure pyridinone intermediate 9c. Selective hydrolysis of the C-5 methyl ester could then be performed by treatment of 9c with LiOH at low temperature, leading to an ∼10:1 ratio of C-5 and C-2 hydrolysis products. Interestingly, LiOH was the preferred reagent, as NaOH and KOH both led to higher amounts of the undesired C-2 ester hydrolysis product. Quenching of the crude reaction mixture and precipitation of the product from warm EtOAc provided compound cabotegravir pyridinone in 61% isolated yield over the 4-step sequence.
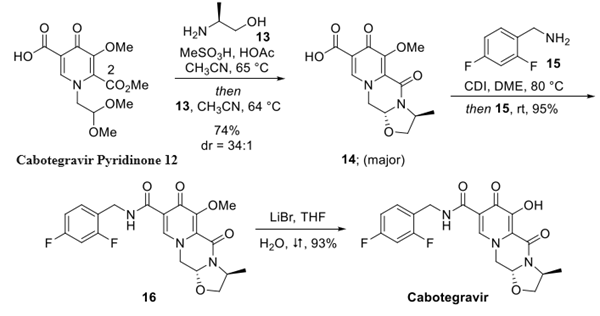
Step 2: Preparation of Cabotegravir by reaction with Cabotegravir Pyridinone
Pyridinone 12 was first treated with MeSO3H and acetic acid in warm acetonitrile, resulting in hydrolysis of the dimethyl acetal to afford the desired aldehyde intermediate, and this was followed by the slow addition of (S)-alaninol (13) to the crude reaction mixture, which after condensing with the transient aldehyde intermediate cyclized with the proximal C-2 ester to generate the core tricyclic structure of cabotegravir in 74% yield over the two steps.
The overall sequence resulted in 14 as the
major product in 34:1 dr, favoring the desired trans product. The
rationale for this selectivity has been described in detail in
previous reports but summarily derives from the comparative stability of the cis- and trans-oxazolidine intermediates,
which lie in equilibrium with their open-chain imine form, and a
more rapid addition of the trans-oxazolidine nitrogen to the C-2
methyl ester due to decreased steric interactions with the methyl
ester in the transition state. While full details are not provided, it
is noted that the diastereoselectivity of 14 can be further
enriched by crystallization at a later point in the synthetic
sequence.
From 14, the final steps of the synthesis include CDI-mediated activation of the carboxylic acid at 80 °C
followed by reaction with fluorobenzyl amine 15, forming amide
16 in 95% yield. Finally, while a variety of conditions were tested
for the demethylation of the enol ether of 16, lithium bromide in
refluxing aqueous THF provided the most scalable set of
conditions for the generation of the drug product, furnishing the
free acid of cabotegravir in 93% yield.
While the free acid form
is required for intramuscular injection, the sodium salt form of
cabotegravir (II), utilized for making the oral form of the drug,
can be generated from the free acid form in high yield (94%) by
treatment with NaOH/EtOH.
You may like
See also
Lastest Price from Cabotegravir (GSK744, GSK1265744) manufacturers

US $240.00/g2025-04-21
- CAS:
- 1051375-10-0
- Min. Order:
- 10g
- Purity:
- 99% Purity (What/sapp: +86 18145728414)
- Supply Ability:
- 1000 Tons/Month

US $15.00-10.00/KG2021-07-02
- CAS:
- 1051375-10-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton