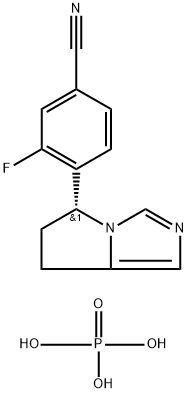
Osilodrostat Phosphate: Synthesis and Introduction
Synthesis of Osilodrostat Phosphate
Osilodrostat Phosphate (LCI 699) is prepared using imidazole salt as raw material and by reduction, alkylation, saponification and other reactions. The specific synthesis steps are as follows:
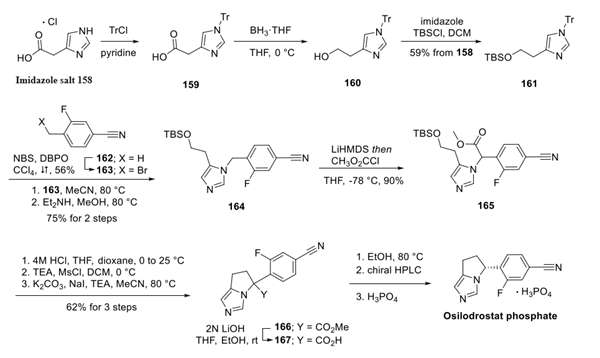
The imidazole salt 158 was first protected with a trityl group to yield tritylamine 159, which was reduced to the primary alcohol 160. Silyl protection of the alcohol provided silyl ether 161 in 59% yield over three steps. Next, alkylation of the imidazole 161 with benzyl bromide 163 (which was prepared from arene 162 using Wohl-Ziegler conditions with NBS and dibenzoyl peroxide (DBPO) in 56% yield) was carried out in refluxing acetonitrile. Subsequent removal of the trityl group using diethylamine afforded benzylamine 164 in 75% yield over two steps. Deprotonation of the benzylic proton followed by methyl chloroformate quench delivered ester 165.
Next, the silyl protecting group was removed upon treatment with acid, and the free alcohol wasthen converted to the mesylate. Exposure to NaI and base afforded the pyrroloimidazole 166 in 62% yield across three steps. Saponification was carried out using aqueous base to afford 167, and thermal decarboxylation afforded the osilodrostat framework as a racemate. The (R)-enantiomer was resolved using chiral HPLC with an acetonitrile mobile phase, and this was followed by phosphate salt formation to provide osilodrostat phosphate with no yield reported for the final four steps.
Introduction of Osilodrostat Phosphate
Osilodrostat phosphate is an orally bioavailable cortisol synthesis inhibitor used to treat hypercortisolism. Osilodrostat inhibits 11β- hydroxylase (CYP11B1), the enzyme responsible for catalyzing the final step of cortisol biosynthesis in the adrenal gland. Osilodrostat was granted USFDA approval with Orphan Designation in 2020 for the treatment of adult patients with Cushing's disease.
Cushing's disease is a rare disease in which circulating cortisol levels are chronically and supraphysiologically elevated. The endogenous causes of Cushing's disease include a cortisol-producing adrenal tumor and adenocorticotrophic hormone (ACTH) hypersecretion secondary to a pituitary tumor. Surgical resection is generally the treatment of choice. As an orally bioavailable therapeutic, osilodrostat provides a novel treatment option that directly targets excessive cortisol production. Discovered and manufactured by Novartis, osilodrostat was originally developed from fadrozole, a structurally related CYP19 inhibitor also developed by Novartis for the treatment of estrogen-dependent breast cancer. With the objective of evaluating the therapeutic benefit of selectively inhibiting aldosterone synthase (CYP11B2) to treat aldosterone-driven pathologies, osilodrostat exhibited equipotent activity against the desired cytochrome, CYP11B2, and the highly homologous CYP11B1.
When dosed in initial human proof-of-concept studies, osilodrostat reduced aldosterone levels while lowering blood pressure and suppressing cortisol production in hypertensive patients. The latter afforded Novartis the opportunity to evaluate the compound as a potential treatment for Cushing's disease. The safety and efficacy of osilodrostat was assessed in a multicenter, 48-week study in patients with persistent or recurrent Cushing's disease. Compared to the placebo, 86% of osilodrostat treated patients were complete responders who had controlled mean urinary free cortisol (mUFC) less than or equal to the upper limit of normal (ULN).