Olmesartan Medoxomil: Pharmacokinetics and Clinical Applications
General Description
Olmesartan medoxomil is a widely used angiotensin II receptor blocker that is rapidly absorbed and efficiently metabolized to its active form, olmesartan. It has various clinical applications in the management of hypertension and related conditions, including heart failure and diabetic nephropathy. Olmesartan medoxomil works by blocking the effects of angiotensin II, promoting vasodilation, and reducing blood pressure levels. It has shown renoprotective properties by reducing proteinuria and slowing the progression of renal disease in diabetic patients. Studies have suggested its potential anti-inflammatory and anti-atherosclerotic effects, making it a valuable tool in reducing the risk of cardiovascular events in high-risk patients. Overall, olmesartan medoxomil is a versatile medication with a proven track record in the management of hypertension and associated cardiovascular conditions.

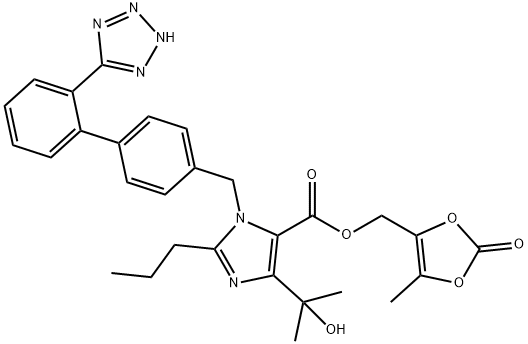
Figure 1. Olmesartan Medoxomil
Pharmacokinetics
Absorption and Distribution
Olmesartan medoxomil is a medication with an absolute bioavailability of 26% after a single oral dose of 20mg in healthy volunteers. When given at doses ranging from 10 to 160mg, Olmesartan medoxomil is rapidly absorbed, with peak plasma concentrations varying from 0.22 to 2.1 mg/L and reached within 1.4 to 2.8 hours post-administration. The area under the plasma concentration-time curve (AUC) for these doses ranged from 1.6 to 19.9 mg•h/L. There is a linear relationship between prodrug dosage and active metabolite peak plasma concentrations (Cmax) and AUC, but not the time to reach peak concentration (tmax). Multiple daily dosages did not significantly increase Cmax values. In patients receiving oral olmesartan medoxomil 80mg once daily, steady-state plasma concentrations were achieved within 3 days. The volume of distribution (Vd) for intravenous doses of 1 to 32mg in healthy male volunteers ranged from 15 to 20L, while a single oral dose of 20mg had a mean Vd of 34.9 ± 20.7L in healthy male volunteers. 1
Metabolism and Elimination
Olmesartan medoxomil undergoes rapid de-esterification in the body to form its active metabolite, olmesartan, which is the only major metabolite identified. No additional metabolites of olmesartan have been detected in humans. The mean terminal elimination half-life of orally administered olmesartan medoxomil varies between 9.8 to 11.4 hours in patients with hypertension and 14.1 to 14.9 hours in healthy male volunteers who received dosages ranging from 20 to 80 mg/day for a duration of 10 days. Urinary excretion of olmesartan accounts for 5 to 12% of the orally administered dose. In animal studies involving dogs and rats, over 90% of the administered olmesartan dose was excreted in the feces within 24 hours. Overall, olmesartan medoxomil is efficiently metabolized to its active form, olmesartan, and eliminated primarily through fecal excretion in animals, while human elimination involves both urinary and fecal routes. 1
Clinical Applications
Olmesartan medoxomil, a widely used angiotensin II receptor blocker (ARB), has various clinical applications in the management of hypertension and related conditions. First and foremost, olmesartan medoxomil is commonly prescribed for the treatment of hypertension in adults and children above six years of age. As an ARB, it works by blocking the effects of angiotensin II, thereby promoting vasodilation and reducing blood pressure levels. It is often used as a first-line agent or in combination with other antihypertensive medications to achieve optimal blood pressure control. In addition to hypertension, olmesartan medoxomil has been found to have beneficial effects in patients with heart failure, diabetic nephropathy, and other cardiovascular conditions. It has demonstrated renoprotective properties by reducing proteinuria and slowing the progression of renal disease in diabetic patients. Furthermore, olmesartan medoxomil may offer additional cardiovascular benefits beyond blood pressure control. Studies have suggested its potential anti-inflammatory and anti-atherosclerotic effects, which could be valuable in reducing the risk of cardiovascular events in high-risk patients. Overall, olmesartan medoxomil is a versatile medication with a proven track record in the management of hypertension and associated cardiovascular conditions, making it a valuable tool in improving patient outcomes and reducing the burden of cardiovascular disease. 2
Reference
1. Schwocho LR, Masonson HN. Pharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjects. J Clin Pharmacol. 2001; 41(5): 515-527.
2. Warner GT, Jarvis B. Olmesartan medoxomil. Drugs. 2002;62(9):1345-1356.
You may like
Related articles And Qustion
Lastest Price from Olmesartan Medoxomil manufacturers

US $0.00-0.00/Kg/Bag2025-10-10
- CAS:
- 144689-63-4
- Min. Order:
- 25Kg/Bag
- Purity:
- CEP/EP
- Supply Ability:
- 20 tons

US $5.00-0.50/KG2025-05-30
- CAS:
- 144689-63-4
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS