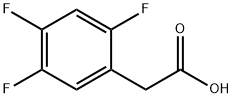
Synthesis and Condensation Reaction of 2,4,5-Trifluorophenylacetic acid
2,4,5-Trifluorophenylacetic acid is a phenylacetic acid derivative that exists as a white to off-white solid powder under ambient conditions. It exhibits significant acidity and excellent chemical stability, being insoluble in water but soluble in alcoholic organic solvents and chloroform. Primarily used as a key intermediate in the synthesis of sitagliptin, a novel drug for treating type II diabetes developed by Merck, 2,4,5-trifluorophenylacetic acid plays a crucial role in the production process. Sitagliptin, the first DPP‑IV inhibitor recently launched by Merck, demonstrates notable therapeutic efficacy for type II diabetes with minimal side effects, along with favorable safety and tolerability profiles, indicating broad market prospects.
Synthesis
Method 1
A patent has reported the development of a synthetic route for 2,4,5-trifluorophenylacetic acid, a key intermediate of sitagliptin, characterized by simple operation, environmental friendliness, and strong practicality, aiming to obtain optimized process parameters. The method involves using methyl 2-(2,5-dicyano-3,4,6-trifluorophenyl)cyanoacetate as the starting material, which undergoes hydrolysis and decarboxylation to yield 2,4,5‑trifluorophenylacetic acid. Factors such as catalyst dosage, acid concentration, solvent selection, and catalyst recovery were investigated for their impact on the reaction. Results showed that in the acid‑catalyzed hydrolysis step, when sulfuric acid concentration reached 60% and the Lewis acid catalyst ferric chloride was used at 20% of the mass of the starting ester, the hydrolysis product yield reached 88%. In the subsequent decarboxylation using copper powder as the catalyst, when the copper powder dosage was 20% of the mass of (2,5‑dicarboxy‑3,4,6‑trifluoro)phenylacetic acid, the maximum yield of the decarboxylation product 2,4,5-trifluorophenylacetic acid achieved 92%. Fresh copper powder was found unfavorable for decarboxylation and required pretreatment, while recovered copper powder and solvent could be reused in the decarboxylation step. In conclusion, this synthetic route offers advantages including readily available materials, easily achievable reaction conditions, and low waste emission. [1]
Method 2
Using 1,2,4-trifluorobenzene as the starting material, 2,4,5-trifluorophenylacetic acid was prepared through chloromethylation, carbonylation, and hydrolysis. Factors such as raw material ratio, temperature, and catalyst dosage were investigated. The results showed that the optimized reaction conditions are as follows: for the synthesis of 2,4,5-trifluorobenzyl chloride, m(C₆H₃F₃):m(polyformaldehyde) = 1.0:0.875–0.865 with a reaction time of 10 h; for the synthesis of 2,4,5-trifluorophenylacetic acid, m(C₇H₄F₃Cl):m(catalyst) = 1:0.275–0.25 at a reaction temperature of 40 °C. Under these conditions, the overall yield of 2,4,5‑trifluorophenylacetic acid exceeds 60%, and the mass fraction of 2,4,5‑trifluorophenylacetic acid reaches over 99%. [2]
Condensation Reaction

Figure1: Condensation Reaction of 2,4,5-trifluorophenylacetic acid
Under an inert atmosphere, add 4 mL of dry-degassed toluene to an oven-dried 25 mL single-neck round-bottom flask, followed by the addition of 2,4,5-trifluorophenylacetic acid (0.5 mmol, 95 mg) and tris(pentafluorophenyl)borane, B(C₆F₅)₃ (0.062 mmol, 12.5 mol%). Stir the resulting mixture at room temperature for 10 minutes, then introduce benzylamine (0.5 mmol, 54 mg) and continue stirring at room temperature for an additional 10 minutes. Attach a condenser to the flask, adding 2 mL of toluene to the condenser to maintain a total toluene volume of 4 mL in the reaction system. Heat the resulting suspension under azeotropic reflux with continuous stirring at 125 °C in an oil bath for three hours. After completion, allow the reaction mixture to cool to room temperature, then remove the solvent under reduced pressure using a rotary evaporator. To the residue, add 10 mL of dichloromethane (DCM), followed by 5 mL of 1 N aqueous hydrochloric acid. Extract the acidic aqueous phase with DCM (3 × 10 mL), combine all DCM extracts, and wash sequentially with water (10 mL) and brine (10 mL). Next, extract the DCM phase with 1 N aqueous sodium hydroxide, and again wash the DCM extract with water (10 mL) and brine (10 mL). Finally, dry the DCM extract over anhydrous sodium sulfate, filter into a round-bottom flask, and concentrate under reduced pressure. [3]
Pharmaceutical Applications
Research has reported a novel synthetic process for sitagliptin utilizing 2,4,5-trifluorophenylacetic acid. The method starts with 2,4,5‑trifluorophenylacetic acid as the raw material, which is condensed with Meldrum’s acid, followed by condensation with NH₂‑BOC. Through asymmetric reduction, the key chiral intermediate (R)‑3‑(tert‑butoxycarbonylamino)‑4‑(2,4,5‑trifluorophenyl)butanoic acid is obtained. Finally, this intermediate is condensed with 3‑(trifluoromethyl)‑5,6,7,8‑tetrahydro‑[1,2,4]triazolo[4,3‑α]pyrazine hydrochloride, followed by deprotection and salt formation to yield the final product sitagliptin. Results showed that sitagliptin was synthesized with an overall yield of 36%. In conclusion, this synthetic route features fewer steps, simple operation, high yield, excellent stereoselectivity, and mild reaction conditions. [4]
Reference
[1] Wang Jianhua, Lang Xuekun, Xiao Wangchuan, et al. Study on the synthesis process of 2,4,5-trifluorophenylacetic acid [J]. Zhejiang Chemical Industry, 2025, 56(2): 21-25.
[2] He Renbao, Wang Yingmei, Jin Yizhong, et al. Synthesis of 2,4,5-trifluorophenylacetic acid [J]. Chemical Production and Technology, 2011, 18: 3.
[3] Gavit, Amit Vinayak; et al, Aryl Borane as a Catalyst for Dehydrative Amide Synthesis, Journal of Organic Chemistry 2025, 90, 2271-2277.
[4] Wu Ting, Liu Yuan, Dong Hongxin, et al. Study on the Synthesis of Sitagliptin, a Therapeutic Agent for Type 2 Diabetes [J]. Chinese Journal of New Drugs, 2015, 24(21): 4.
You may like
See also
Lastest Price from 2,4,5-Trifluorophenylacetic acid manufacturers

US $0.00/kg2025-07-11
- CAS:
- 209995-38-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 209995-38-0
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt