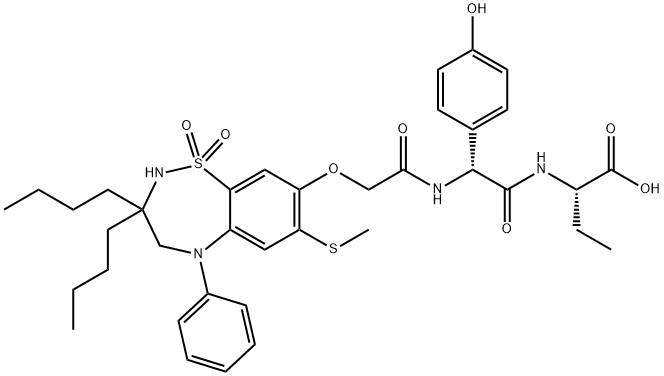
Odevixibat: Synthesis and Application
Synthesis of Odevixibat
Odevixibat is synthesised in two steps by chemical reaction using enfumafungin as a raw material. The specific synthesis steps are as follows:
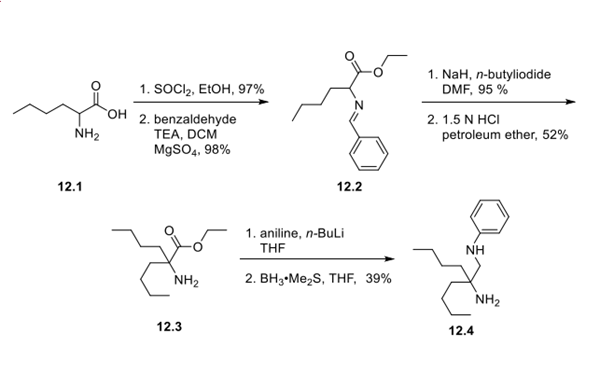
Step 1: Synthesis of Amine Fragment
To access the first fragment, 2-aminohexanoic acid 12.1 was esterified and treated with benzaldehyde to form imine 12.2, presumably to protect the free nitrogen. Alkylation with n-butyl iodide followed by acidic removal of benzylamine gave amino ester 12.3. Transamidation with aniline and subsequent borane reduction afforded amine fragment 12.4.
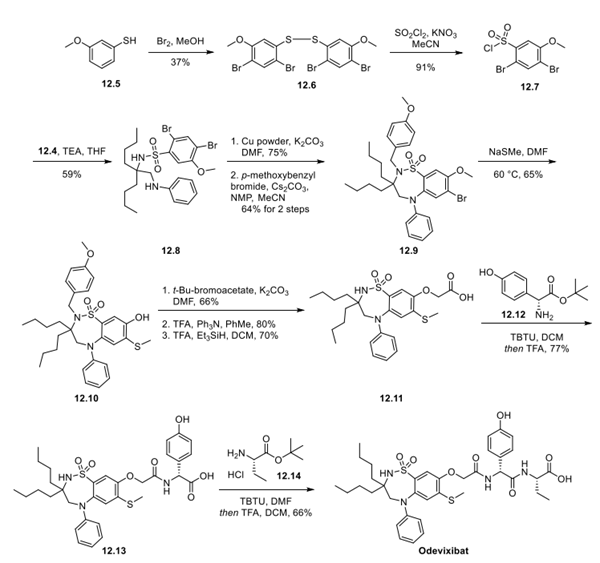
Step 2: Preparation of Odevixibat
Bis-bromination of 3-thioanisole 12.5 with bromine in MeOH afforded the dibrominated disulfide 12.6, which was subjected to a one-pot oxidative conversion to sulfonyl chloride 12.7 in good yield. An SN2 reaction between tertiary amine 12.4 and aryl sulfonyl chloride 12.7 gave 12.8, which underwent ring formation by treatment with copper powder and K2CO3 in DMF. Protection of the sulfonamide nitrogen with para-methoxybenzyl bromide preceded demethylation and installation of thioether via SNAr to afford phenol 12.10. The peptidic side chain was then constructed, beginning with O-alkylation with t-butylbromoacetate, followed by protecting group removal under acidic conditions to afford 12.11. Two consecutive amidation reactions were performed with activator TBTU. Amine 12.12 was coupled to 12.11, followed by removal of the t-butyl group with TFA. The second amidation with amine 12.14 followed by in situ removal of the t-butyl group delivered odevixibat in 66% yield.
Application of Odevixibat
Odevixibat is a once-daily small-molecule inhibitor of the ileal bile acid transporter. The drug was developed by Albereo Pharma, Inc. and approved for the treatment of various cholestatic diseases such as progressive familial intrahepatic cholestasis (PFIC), bilary atresia, and Alagille syndrome. Cholesteric liver disease can occur when impaired bile formation and/or flow results in accumulation of bile components within the liver, resulting in liver inflammation, fibrosis, and liver injury. These disease states can lead to jaundice, general fatigue, and pruritus, and in many cases liver transplant is required.
Odevixibat reduces the levels of bile acid in the plasma/serum by reducing uptake in the distal ileum as well as by increasing colon clearance. Odevixibat received USFDA approval for the treatment of pruritus in PFIC patients and EU approval for PFIC patients aged ≥6.128 Odevixibat was granted orphan designations in the US and EU for the treatment of Alagille syndrome, biliary atresia, and primary biliary cholangitis.