The introduction of Siponimod
Description
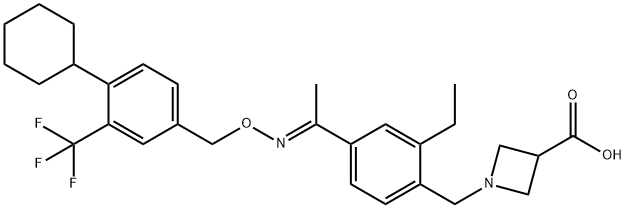
Siponimod (Mayzent®; BAF-312) is an oral selective sphingosine 1-phosphate receptor subtypes 1 and 5 (S1PR1,5) modulator being developed by Novartis Pharmaceuticals for the treatment of multiple sclerosis (MS) and intracerebral haemorrhage.
Mode of action
The relapsing form of MS includes clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease in adults. S1P regulates physiological processes such as lymphocyte trafficking, cardiac function, vascular development, and inflammation. Siponimod binds with nanomolar potency to S1P, located on the extracellular region of immune cells, blocking its regular action. It can cross the blood–brain barrier (BBB) and selectively binds to S1PR1 and S1PR5 expressed by several cell populations of the central nervous system (CNS), including microglia. The therapeutic mode of action involves the downmodulation of S1P receptors on T-lymphocytes, sequestered within secondary lymphoid tissues, preventing recirculation into the central nervous system. This reduces immune attacks on nerve cells in the brain and spinal cord.
Synthesis method
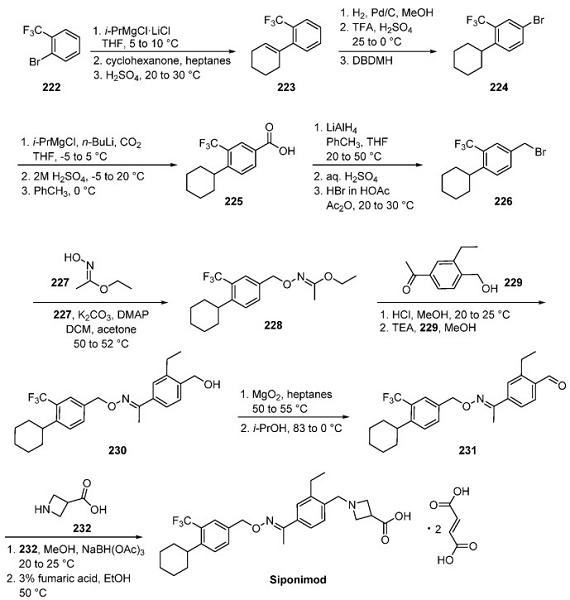
The most likely process-scale synthesis of siponimod, illustrated in Scheme 1, was described by Novartis in a 2013 patent, although no yields were reported. Aryl bromide 222 was converted to olefin 223 by means of Grignard addition followed by acid-mediated elimination. Hydrogenation of this olefin and subsequent bromination afforded bromoarene 224. Conversion of this arene to the corresponding benzyl bromide was facilitated through a carbonylation reaction, which was followed by oxidation state adjustment and subjection to HBr to furnish benzyl bromide 226. Subjection to oxime 227, followed by a substitution reaction with ketone 229, led to the formation of oxime ether 230. Oxidation of the benzyl alcohol to the corresponding benzaldehyde preceded reductive amination with azetidine 232 to complete the synthesis of siponimod, which was then treated with ethanolic fumaric acid to provide the target product as the bis(fumarate) salt.
References
[1] Al-Salama, Zaina T. “Siponimod: First Global Approval.” Drugs 79 9 (2019): 1009–1015.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from BAF-312(SiponiMod) manufacturers

US $0.00-0.00/KG2025-04-16
- CAS:
- 1230487-00-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5000

US $0.00-0.00/g2024-11-01
- CAS:
- 1230487-00-9
- Min. Order:
- 1g
- Purity:
- 98%
- Supply Ability:
- 10kgs