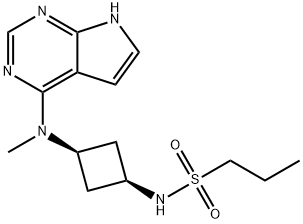
Abrocitinib: Synthesis and Application
Synthesis of Abrocitinib
Abrocitinib is synthesised using ketoester as a raw material by chemical reaction. The specific synthesis steps are as follows:
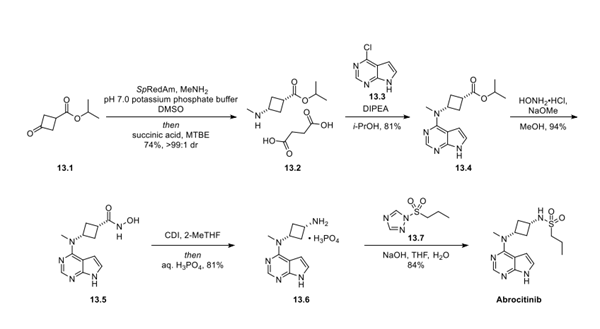
The synthesis began with commercially available ketoester 13.1, which underwent an enzymatic reductive amination with methyl amine to form aminocyclobutane 13.2, isolated as the succinate salt in 74%yield and >99:1 cis:trans ratio. Exposure of the amine to pyrrolopyrimidine 13.3 afforded the SNAr product 13.4 in 81% yield. Conversion of the ester to the corresponding hydroxamic acid 13.5 proceeded in 94% yield. Activation of the hydroxamic acid with N,N′-carbonyl-diimidazole (CDI) induced the desired Lossen rearrangement, which after acidic hydrolysis with phosphoric acid resulted in the cis-cyclobutanediamine salt 13.6, isolated in 81% yield. Finally, sulfonylation of the amine with sulfonyl triazole 13.7 occurred in 84% isolated yield to afford abrocitinib.
Application of Abrocitinib
Abrocitinib is an orally available small-molecule Janus Kinase 1 (JAK1) inhibitor developed by Pfizer and approved in September 2021 in the UK and Japan for the treatment of moderate to severe atopic dermatitis. Atopic dermatitis is an inflammatory skin condition that often presents in infancy or childhood, with between 2.7 and 20.1% of children and between 2.1 and 4.9% of adults having the affliction. Abrocitinib functions by inhibiting cytokine-induced phosphorylation of JAK-signal transducer and activator of transcription (STAT) pairs that include JAK1.
You may like

US $0.00/kg2025-06-07
- CAS:
- 1622902-68-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00/g2024-11-19
- CAS:
- 1622902-68-4
- Min. Order:
- 1g
- Purity:
- 98% HPLC
- Supply Ability:
- 1kg