Nonivamide: Pharmacological Actions and Biosynthesis Method
General Description
Nonivamide is a pharmacologically significant capsaicinoid with applications in riot control and agriculture, known for its role as an agonist of the TRPV1 receptor, leading to pain perception and potential ocular damage. Research indicates that nonivamide inhibits the proliferation of human corneal epithelial cells by inducing cell cycle arrest and oxidative stress, particularly at subtoxic doses. Furthermore, advancements in nonivamide biosynthesis through microbial cell factories utilize renewable substrates such as plant oils and vanillylamine, resulting in improved yields. This biotechnological approach presents a more sustainable and efficient method for producing nonivamide compared to traditional extraction techniques.

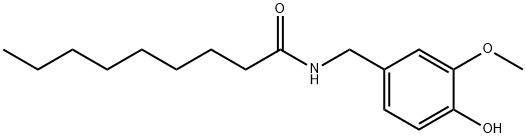
Figure 1. Nonivamide
Pharmacological Actions
Nonivamide is a chemical compound known for its role as an agonist of the transient receptor potential vanilloid type 1 receptor, commonly referred to as TRPV1. This receptor is primarily involved in pain perception and contributes to various physiological responses to environmental stimuli. Beyond its pharmacological applications, nonivamide is frequently utilized as a riot control agent, police incapacitant spray, and pesticide. Its use, while generally considered non-fatal, can lead to significant ocular discomfort and injuries when exposed to the eyes. Despite its prevalence in situations requiring crowd control, there has been scant research focusing on the specific impact of nonivamide on human corneal epithelial cells, which are crucial for maintaining the health of the eye. 1
Mechanisms of Action
Recent studies have highlighted that nonivamide at subtoxic doses of 100 μM and 200 μM significantly inhibits the proliferation of human corneal epithelial cells, termed HCE-T cells. The underlying mechanisms by which nonivamide exerts its effects include inducing cell cycle arrest and generating oxidative stress. The compound activates TRPV1 receptors, facilitating an influx of calcium ions into the cells. This influx of calcium ions is a critical factor contributing to the oxidative stress observed in these cells. Additionally, the presence of nonivamide leads to alterations in protein expressions, notably an increase in p21 and a decrease in cyclin D1, which are key regulators of the cell cycle. These changes collectively hinder the normal proliferation of HCE-T cells, indicating that nonivamide has significant implications for cell viability. 1
Implications of Nonivamide-Induced Corneal Injury
The implications of nonivamide's effects on human corneal epithelial cells are particularly concerning given the widespread use of this compound in various applications. The decrease in mitochondrial membrane potential, along with the oxidative stress and cell cycle arrest induced by nonivamide, provides a clear pathway for corneal toxicity. These findings highlight the potential for serious ocular damage associated with exposure to nonivamide. Understanding the specific toxicological effects and mechanisms of action of nonivamide is crucial for informing safety guidelines and handling practices, especially in contexts where exposure to nonivamide is likely. In summary, the research not only elucidates the pharmacological actions of nonivamide but also stresses the need for caution in its use, particularly concerning the health and safety of the ocular surface. 1
Biosynthesis Method
Overview of Nonivamide Biosynthesis
Nonivamide, a capsaicinoid, is primarily produced through biotechnological methods utilizing microbial cell factories, which offer a more efficient alternative to traditional plant extraction. The biosynthesis of nonivamide involves the use of renewable substrates such as plant oils and vanillylamine. In a notable study, nonivamide was synthesized using nonanoic acid and vanillylamine in *Escherichia coli* through the heterologous expression of specific genes responsible for the amide-forming pathway. Key enzymes involved in nonivamide production were identified, and conditions for optimal biotransformation were strategically improved, ultimately achieving a nonivamide titer of 0.5 grams per liter. 2
Advancements in Nonivamide Production
To enhance the biosynthesis process of nonivamide, researchers integrated a pathway that converts oleic acid, a common component of plant oils, into nonanoic acid. This innovative approach allowed for the utilization of olive oil, combined with vanillylamine, as substrates for synthesizing nonivamide. The resulting yield from this method reached approximately 10.7 milligrams per liter. These advancements in nonivamide production highlight the potential of constructing highly efficient microbial systems for the biosynthesis of capsaicinoids, paving the way for more sustainable sources of these valuable compounds in food, pharmaceutical, and agricultural applications. Through the optimization of biotransformation processes, nonivamide can be produced more economically and sustainably, benefiting various industries. 2
References:
[1] JIANZHONG GE . Production of capsaicinoid nonivamide from plant oil and vanillylamine via whole-cell biotransformation[J]. Bioresource Technology, 2023, 390. DOI:10.1016/j.biortech.2023.129883.[2] HAIYUN LI. Nonivamide inhibits proliferation of human corneal epithelial cells by inducing cell cycle arrest and oxidative stress[J]. Toxicology, 2023, 500. DOI:10.1016/j.tox.2023.153674.
You may like
Lastest Price from Nonivamide manufacturers

US $1.00/kg2025-04-21
- CAS:
- 2444-46-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-04-21
- CAS:
- 2444-46-4
- Min. Order:
- 1KG
- Purity:
- ≥98% HPLC
- Supply Ability:
- 1000KG