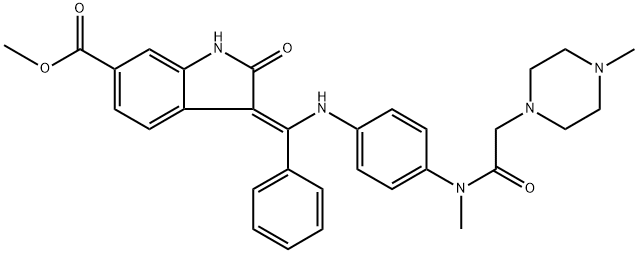
Nintedanib: Uses, Mechanism of Action and Side Effects
What is Nintedanib?
Nintedanib is a small molecule tyrosine kinase inhibitor (TKI) that binds to a family of growth factor receptors and blocks the proliferation of fibroblasts. It not only reduces the ongoing fibrotic process but also slows the progression of long-term damage.In 2014, Nintedanib was approved by the FDA for the treatment of idiopathic pulmonary fibrosis (IPF). Then, in 2020, the FDA approved Ofev (nintedanib) oral capsules for the treatment of patients with chronic fibrotic (scarring) interstitial lung disease (ILD) with a progressive phenotype (features). This is the first drug approved by the FDA to treat this type of fibrotic lung disease that worsens over time.
Mechanism of Action
Nintedanib is a small molecule indolinone derivative that binds tyrosine kinase receptors. Its mechanism of action is to limit neoangiogenesis by inhibiting a number of growth factors (e.g. *FGFR, VEGFR, PDGFR, CSF1R, and FLT3).Nintedanib competitively binds to the intracellular adenosine triphosphate (ATP) binding site of growth factor receptors, preventing autophosphorylation and blocking downstream signalling cascades. In addition, Nintedanib directly blocks non-receptor tyrosine kinases such as Src and Lck, thereby preventing fibroblast activation. Thus, Nintedanib ultimately inhibits fibroblast proliferation and migration, effectively attenuating angiogenesis within the lung. The reduction in fibrosis and angiogenesis helps to halt the progression of ILD, IPF and related complications such as pulmonary hypertension.
Side Effects
The most common side effects reported in the Ofev clinical trial were diarrhea, nausea, stomach pain, vomiting, liver problems, decreased appetite, headache and weight loss. Ofev is not recommended for patients with moderate or severe hepatic (liver) impairment. Elevated liver enzymes and drug-induced liver injury and gastrointestinal disorders have occurred among people taking Ofev. It may also cause embryo-fetal toxicity that can result in fetal harm, arterial thromboembolic events (blood clots), bleeding and gastrointestinal perforation (hole formation). P-glycoprotein and CYP3A4 inhibitor drugs, including ketoconazole and erythromycin, may increase nintedanib exposure, and patients taking these inhibitors with Ofev should be closely monitored.
References:
[1] Nintedanib[J]. Reactions Weekly, 2022. DOI:10.1007/s40278-022-18502-5.
[2] Nintedanib associated with proteinuria[J]. Reactions Weekly, 2021, 139 1: 4-4. DOI:10.1007/s40278-021-98608-2.
You may like
See also
Lastest Price from Nintedanib manufacturers
US $0.00/kg2025-09-20
- CAS:
- 656247-17-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $5.00-0.50/KG2025-06-05
- CAS:
- 656247-17-5
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS