Nepafenac:Pharmacology and synthesis
Introduction
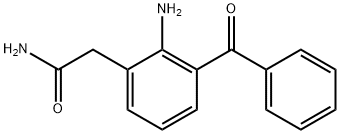
Nepafenac (Figure 1) is the international common accepted name for 2-amino-3-benzoyl-phenylacetamide, and has empirical formula of C15H14N2O2, and a molecular weight of 254.28g/mol. Nepafenacis a non-steroidal anti-inflammatory prodrug usually sold as a prescription eye drop. It is used to treat pain and inflammation associated with cataract surgery. Nepafenac is insoluble in aqueous solution, a suspension of the drug was prepared in PBS, it was important to monitor the intraocular pressure of the eye following injection of the suspension, and there was no significant change in IOP at all time points following intra-cameral delivery. Nepafenac as a new generation of non steroid anti-inflammatory drugs (NSAID) precursor was approved by FDA, listed on the first eye ophthalmic NSAIDS precursor pharmaceutical preparations in 2005; and its eye ophthalmic suspension liquid (commodity name Nevanac, Alcon Company), mainly used in the treatment of cataract surgery pain and inflammation. European Medicines Agency (EMA) has approved the new indications of nepafenac (developed by alcon), which can be used to relieve pain and inflammation of diabetic patients after cataract surgery, and reduce the risk of diabetic macular edema after cataract surgery.[1]

Pharmacology and Pharmacodynamics
Nepafenac is commercially available in two concentrations 0.1% and 0.3%.[2] The pharmacology of nepafenac is after ocular drug delivery, rapidly passing through the cornea, transformed to Amfenac under the action of eye tissue hydrolase, and it can quickly reach the targeted point and play a role. Nepafenac has several advantages over other ophthalmic NSAIDs. First, it has a unique prodrug structure. Prodrugs are the inactive form of a drug that converts to an active form after metabolic conversion in the body. After topical ocular dosing, nepafenac quickly penetrates the cornea and is converted to amfenac, which is a potent NSAID with a safe therapeutic profile. Because its prodrug mechanism action maximizes bioactivation of nepafenac to amfenac in the cornea, iris-ciliary body, andretina/choroid, the literature supports the classification of nepafenac as the first and only target-specific NSAID for inhibiting prostaglandin formationin the anterior and posterior segments of the eye. Importantly, this prodrug structure may reduce the risk of surface complications, because the prodrug penetrates quickly through the cornea and ocular surface tissues.[1]
Low but quantifiable plasma concentrations of nepafenac and amfenac were observed in the majority of subjects 2 and 3 hours postdose, respectively, following bilateral topical ocular TID dosing of nepafenac ophthalmic suspension, 0.1%. The mean steady-state Cmax for nepafenac and for amfenac were 0.310 ± 0.104 ng/ml and 0.422 ± 0.121 ng/ml, respectively, following ocular administration.[4]
Nepafenac compared with the traditional non-steroidal anti-inflammatory drug, has the advantages of a stronger penetration, a stronger target function, and a smaller side effects, etc. The People's Republic of China has approved the application of Alcon (China) ophthalmic product co., about clinical registration of nepafenac eye drops, and not yet on the market in domestic at present. [5]
Synthesis of Nepafenac
Dai‘s article is a study on the synthesis route and synthesis process, aimed at improving the production process which started from 2-Aminobenzophenone for the synthesis of 2-amino-3-benzoyl-phenylacetamide to enhance the product’s yield. The cost of materials, catalyst, temperature, chloride reagent, purification of solvent, reaction conditions and post-processing were determined for its industrialization. The improved synthetic methods of nepafenac were researched, and the procedure is more helpful for the industrial production. The experiment results showed as the following: The foul smell of sodium thiophenolate is larger and the price of it is very higher, not using this route. The materials of methyl (methylthio) acetate or ethyl (methylthio) acetate can not be easy to buy in the domestic market. Although started from methanethiol sodium salt and methyl chloroacetate for the VI synthesis of methylthio acetamide has a low yield, the raw materials are easily available and cheap, and the quality of methylthio acetamide is more stable (>99.9%), and the synthetic smell of this reaction is bigger, suggesting small-scale production or purchase; There is not a great deal of difference between the catalytic effect of tetrabutyl ammonium bromide and tetramethyl ammonium bromide. The chlorinating agent of tert-butyl hypochlorite has complex preparation, and the property is not stable, the detection results at any time to change, determining not to use this chlorinating agent; There are some great advantage of high process yield, stable quilty to use the chlorinating agent of N-Chlorosuccinimide; Compared with tert-butyl hypochlorite, the chlorinating agent of sulfochlorides has overcome the drawbacks of low yield and has not been easy to store; Compared with N-Chlorosuccinimide, the chlorinating agent of sulfochlorides has some merits and demerits, such as the merits of more economic advantage, the demerits of the requirement of temperature in the process and leading to the increase of energy consumption, so the process of using sulfochlorides has yet to be further research. To ethanol was added the raw nepafenac (ethanol/nepafenac=40/1), heated to completely dissolved by thermostat oil bath pot, decolorized by activated carbon, naturally cooled to 0℃ and stirred to separate out enough light yellow needle-like crystal (HPLC showing the purity of product reached 99.8%), obtaining the yield of 80%. The optimized synthesis route of nepafenac has the advantages of the following: mild reaction conditions, good reproducibility, and the quality of products are stable. The results for the synthesis of nepafenac have an impotant guiding significance for industrial production, and the product yield has a substantial increace.
Conclusions
Nepafenac is a unique topical ophthalmic nonsteroidal prodrug that converts to amfenac in the iris, ciliary body, retina, and choroid. Amfenac effectively inhibits prostagland in synthesis and protein extravasation,stabilizing the blood-retina and blood-aqueous barriers. Its prodrug mechanism is unique among ocular NSAIDs and has been shown to reduce and prevent inflammation in the anterior segment in humans,and in the posterior segment in experimental models.Because the breakdown of the blood-retina barrier has been found to be related to the development of CME, nepafenac may play a role in the prevention of CME and heralds a paradigm shift in how we use topical NSAIDs in cataract and lens related refractive surgery.
References
[1] Lane SS. Nepafenac: a unique nonsteroidal prodrug. Int Ophthalmol Clin. 2006;46(4):13-20. doi:10.1097/01.iio.0000212129.53019.49
[2] Bezatis A, Georgou I, Dedes J, Theodossiadis P, Chatziralli I. Nepafenac in cataract surgery. Clin Exp Optom. 2022;105(3):263-267. doi:10.1080/08164622.2021.1945412
[3] Jha R, Sur V, Bhattacharjee A, et al. Intracameral Use of Nepafenac: Safety and Efficacy Study. Curr Eye Res. 2018;43(5):630-638. doi:10.1080/02713683.2017.1408129
[4] https://go.drugbank.com/drugs/DB06802
[5] Guang DL. Improvement in the Synthesis of 2-amino-3-benzoyl-phenylacetamide[D].University of Jinan,2013.
You may like
Lastest Price from Nepafenac manufacturers
US $0.00-0.00/kg2025-08-21
- CAS:
- 78281-72-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/Kg/Bag2025-04-21
- CAS:
- 78281-72-8
- Min. Order:
- 10g
- Purity:
- 98%-101.5%
- Supply Ability:
- 10kg