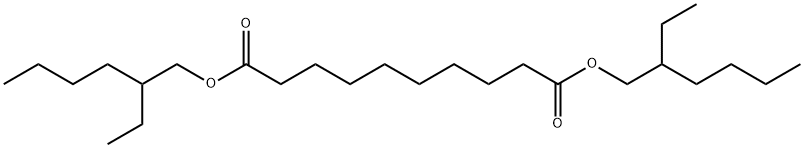
Bis(2-ethylhexyl) Sebacate: Endocrine Disruption in Fish, Oxidation Product Evolution, and Effects on Ion-Selective Membranes
Bis(2-ethylhexyl) sebacate(DOS)is a non-polar plasticizer used in the formation of thin plasticized polyvinyl chloride (PVC)based membranes.It is also used in organic synthesis,pharmaceuticals,agrochemicals,and dyestuff fields.Additionally,it is widely used in preparing ion-selective electrodes.

Effects of non-phthalate plasticizer bis(2-ethylhexyl) sebacate (DEHS) on the endocrine system
Water pollution due to plasticizers is one of the most severe environmental problems worldwide. Phthalate plasticizers can act as endocrine disruptors in vertebrates. In this study, we investigated whether the non-phthalate bis(2-ethylhexyl) sebacate (DEHS) plasticizer can act as an endocrine disruptor by evaluating changes in the expression levels of thyroid hormone-related, reproduction-related, and estrogen-responsive genes of Japanese medaka (Oryzias latipes) exposed to the plasticizer. Following the exposure, the gene expression levels of thyroid-stimulating hormone subunit beta (tshβ), deiodinase 1 (dio1), and thyroid hormone receptor alpha (trα) did not change. Meanwhile, bis(2-ethylhexyl) sebacate suppressed dio2 expression, did not induce swim bladder inflation, and eventually reduced the swimming performance of Japanese medaka. These findings indicate that DEHS can potentially disrupt the thyroid hormone-related gene expression and metabolism of these fish. However, exposure to bis(2-ethylhexyl) sebacate did not induce changes in the gene expression levels of kisspeptin 1 (kiss1), gonadotropin-releasing hormone (gnrh), follicle-stimulating hormone beta (fshβ), luteinizing hormone beta (lhβ), choriogenin H (chgH), and vitellogenin (vtg) in a dose-dependent manner. This is the first report providing evidence that DEHS can disrupt thyroid hormone-related metabolism in fish.[1]
Bis-(2-ethylhexyl) sebacate (DEHS), a non-phthalate plasticizer, is used as a lubricant in reaction motors and vacuum pumps (PubChem CID; 31218). Some studies reported that DEHS was detected in groundwater in Beijing and Tianjin, China (undetectable to <33 ng/L) and Hanoi and Ho Chi Minh, Vietnam (0.02–0.13 μg/L) as well as in the domestic indoor air (0.007–0.13 μg/m3) in Sapporo, Japan. Phthalate plasticizers are well known to act as endocrine disruptors in mammals, birds, and fish. However, it remains unknown whether bis(2-ethylhexyl) sebacate also has the same effect in teleosts. DEHP and DEHS have similar chemical structures as they both contain ethylhexyl alcohol. In addition, data regarding DEHS-induced toxicity are limited in the literature, except for reports on DEHS-induced liver peroxisome proliferation in rats and a lack of DEHS-induced acute toxicity in HeLa cells. Therefore, this study aimed to clarify whether the non-phthalate plasticizer bis(2-ethylhexyl) sebacate disrupts the endocrine system of Japanese medaka, particularly the expression of reproduction-related, thyroid hormone-related, and estrogen-responsive genes of these fish. Additionally, swim bladder inflation, eye size, total body length, and swimming performance (all related to thyroid hormone activity) were assessed. The findings of this study contribute valuable new knowledge on the potential risk of a non-phthalate plasticizer and will be useful for dealing with global plastic pollution.
This study confirms that bis(2-ethylhexyl) sebacate has the potential to exert endocrine-disrupting activity in Japanese medaka (Oryzias latipes). Additionally, DEHS suppressed dio2 expression, caused swim bladder inflation failure, and reduced swimming performance. These findings indicate that DEHS has the potential to disrupt thyroid hormone-related metabolism in fish. However, exposure to bis(2-ethylhexyl) sebacate did not alter the expression levels of reproduction-related and estrogen-responsive genes, suggesting that bis(2-ethylhexyl) sebacate may not disturb the endocrine-related aspects of the reproductive system in fish.
The statistical evolution of multiple generations of oxidation products
The heterogeneous reactions of hydroxyl radicals (OH) with squalane and bis(2-ethylhexyl) sebacate (BES) particles are used as model systems to examine how distributions of reaction products evolve during the oxidation of chemically reduced organic aerosol. A kinetic model of multigenerational chemistry, which is compared to previously measured (squalane) and new bis(2-ethylhexyl) sebacate experimental data, reveals that it is the statistical mixtures of different generations of oxidation products that control the average particle mass and elemental composition during the reaction. The model suggests that more highly oxidized reaction products, although initially formed with low probability, play a large role in the production of gas phase reaction products. In general, these results highlight the importance of considering atmospheric oxidation as a statistical process, further suggesting that the underlying distribution of molecules could play important roles in aerosol formation as well as in the evolution of key physicochemical properties such as volatility and hygroscopicity.[2]
The influence of plasticizer and lipophilic background electrolyte
The dissociation constants in ion-selective membranes are dominated by the dielectric constant of the membranes. The simplest way to change the dielectric properties of PVC membranes is by changing the membrane plasticizer. Bis(2-ethylhexyl) sebacate (DOS) and 2-nitrophenyl octyl ether (o-NPOE) are representatives of low and high dielectric constant plasticizers with dielectric constants of 4 and 24, respectively, in their pure forms. Bis(2-ethylhexyl) sebacate and o-NPOE plasticized PVC membranes have much higher dielectric constants. Values ranging between 16 and 20 and between 37 and 45 were calculated from impedance spectroscopy data, respectively. In general, o-NPOE plasticized membranes have smaller resistances than DOS plasticized ones. This reduced resistance is because the concentration of ionic impurities in o-NPOE is much larger than in DOS. In addition, the degree of dissociation of electrolytes is larger inside the high dielectric constant o-NPOE plasticized membranes compared to Bis(2-ethylhexyl) sebacate plasticized membranes with low dilelectric contant (e.g., TDDMACl-based anion-selective membranes). Finally, the diffusion coefficients in o-NPOE are also somewhat larger.[3]
A large difference between the effects of added lipophilic electrolytes on the resistivities of bis(2-ethylhexyl) sebacate and o-NPOE plasticized membranes was also observed with TDDMACl-based anion-selective membranes. Upon the incorporation of the same amounts of ETH 500 into these membranes, the increase in the absolute conductivity was much larger with o-NPOE than with bis(2-ethylhexyl) sebacate plasticized membranes. However, the authors concluded that ETH 500 has a greater effect on the conductivity of DOS than of o-NPOE plasticized membranes. Indeed, the increase in the membrane conductivity relative to the conductivity of a membrane containing only TDDMACl was larger in bis(2-ethylhexyl) sebacate plasticized membranes. However, the large relative increase in the membrane conductivity was due to the originally very low conductivity of the TDDMACl-containing DOS plasticized membrane, since TDDMACl does not dissociate very much in bis(2-ethylhexyl) sebacate. This conclusion is supported by the fact that conductivity increase per unit of added ETH 500 is actually more than 10 times higher in membranes plasticized with o-NPOE than DOS. Furthermore, the authors concluded that the ions of ETH 500 “hardly participate in the conductivity process” and the increased membrane conductivity was the consequence of increased water content in the ETH 500-loaded membranes. However, in light of the results in this paper and the new interpretation of the results of Legin et al., it appears more likely that the ions of the background electrolyte are themselves the primary source of the experimentally recorded conductivity increase.
The effects of membrane plasticizer and a background electrolyte incorporated into ion-selective membranes were analyzed in light of a theory that was developed to quantify free ionophore, ion-ionophore complex, and anion diffusion coefficients inside ion-selective membranes, and thereby to quantify the effects of the plasticizer and background electrolyte on the diffusion coefficients. The conductivity of the studied membranes increased with the background electrolyte concentrations in both bis(2-ethylhexyl) sebacate and o-NPOE plasticized membranes. The increase in conductivity due to the background electrolyte reduces migration inside the membrane. Changes in the contribution of migration in the overall transport produce changes in the concentration profiles of the ion-ionophore complex. The theory accurately predicts these changes in the concentration profiles, but somewhat underestimates the experimental voltage changes, especially for bis(2-ethylhexyl) sebacate -plasticized membranes.
References
[1]Horie Y, Nomura M, Ramaswamy BR, Harino H, Yap CK, Okamura H. Effects of non-phthalate plasticizer bis(2-ethylhexyl) sebacate (DEHS) on the endocrine system in Japanese medaka (Oryzias latipes). Comp Biochem Physiol C Toxicol Pharmacol. 2023 Feb;264:109531.
[2]Wilson KR, Smith JD, Kessler SH, Kroll JH. The statistical evolution of multiple generations of oxidation products in the photochemical aging of chemically reduced organic aerosol. Phys Chem Chem Phys. 2012 Jan 28;14(4):1468-79.
[3]Zook JM, Langmaier J, Lindner E. Current-polarized ion-selective membranes: The influence of plasticizer and lipophilic background electrolyte on concentration profiles, resistance, and voltage transients. Sens Actuators B Chem. 2009 Mar 2;136(2):410-418.
You may like
See also
Lastest Price from Bis(2-ethylhexyl) sebacate manufacturers
US $0.00-0.00/KG2025-11-25
- CAS:
- 122-62-3
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00/PCS2025-04-21
- CAS:
- 122-62-3
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt