N-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester
General description
N-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester is the 6th synthesis intermediate of remdesivir. Remdesivir was developed by Gilead of the United States. The original research and development intention was to resist Ebola virus (EBOV) [1. However, with the deepening of research, it was found that the antiviral effect of Remdesivir is not limited to filoviruses such as Ebola virus, and it also has an inhibitory effect on coronaviruses and other viruses. On May 1, 2020, the FDA issued an emergency use authorization for remdesivir to treat new coronary pneumonia. Japan approved the use of the drug on May 7th. As Japan's first new coronary pneumonia treatment drug, it will be used for the treatment of severely ill patients with new coronary pneumonia.
Application
Remdesivir is a nucleoside analog being developed by Gilead Sciences. It was reported to have anti-RNA virus activity in 2012. Since then, more in vitro and animal experiments have proved that it has the treatment of Ebola. Viral diseases, Middle East respiratory syndrome and other RNA virus infections have promising applications, but they are not as effective as monoclonal antibodies in clinical research on the treatment of Ebola virus disease. On January 31, 2020, the "New England Journal of Medicine" published an online paper, introducing for the first time the rapid reduction of clinical symptoms of patients with novel coronavirus pneumonia (COVID-19) in the United States after using Radixivir. For this, we can see the importance of N-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester as the intermediate of remdesivir.
Synthesis
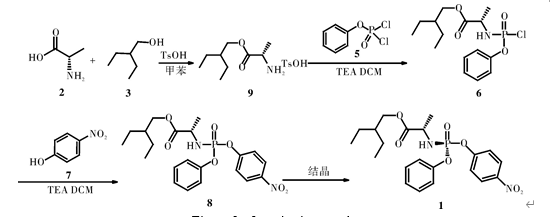
Route 1: L-alanine (compound 2) and 2-ethyl-1-butanol (compound 3) are catalyzed by p-toluenesulfonic acid, dehydrated in toluene under reflux for 14 h, concentrated, and crystallized with ether/isohexane Compound 9 (p-toluenesulfonate) was obtained. In dichloromethane, triethylamine was used as an acid binding agent to react with phenyl dichlorophosphate (compound 5) and p-nitrophenol (compound 7) to obtain compound 8, which was used to prepare Purification by chromatography, crystallization of isopropyl ether/isohexane to obtain target compound 1, with a total yield of 21.7%. The synthetic route is shown in Figure 1. This route requires the use of preparative chromatography for purification, a large amount of mixed solvents are used in the process, the total yield is low, and it is not suitable for industrial production.
Figure 1 of synthesis route 1
Route 2: Using Boc-protected L-alanine (compound 10) as starting material, esterification with 2-ethyl-1-butanol (compound 3) under the catalysis of TEA and DMAP, in dioxane , The Boc protecting group was removed with HClI, dichloromethane was the solvent, and triethylamine catalyzed by reacting with phenyl dichlorophosphate (compound 5) at -78 °C to produce compound 6. The synthetic route is shown in Figure 2. Route 2 is one step more than route 1 to remove Boc, and needs to be reacted at -78°C, which is not conducive to industrial production, and there is no report of subsequent reactions in the literature, and the yield cannot be calculated.
Figure 2 of synthesis route 2
The synthetic process of compound 1 is improved on the basis of Route 1. Using the starting material 2-ethyl-1-butanol (compound 3) as both reactant and solvent, SOCly catalyzed esterification to obtain compound 4 (hydrochloride) in one step; using THF as solvent and DIPEA as acid binding agent , Followed by reaction with phenyl dichlorophosphate (compound 5) and p-nitrophenol (compound 7) to obtain compound 8, which was crystallized by isopropyl ether to obtain the target product with a total yield of 40%. The synthetic route is shown in Figure 3. The improved route is easy to operate, high yield, high purity of the target product (99.6%), high chiral purity (chiral purity 99.8%), suitable for industrial production. By investigating the amount of catalyst, reaction temperature, crystallization solvent and crystallization temperature, the optimal process conditions were determined, and its structure was characterized by 1H-NMR and MS.
Figure 3 of synthesis route 3
Reference
1.Shi Yueling,Huang Guangdong,He Minhuan,et al.Study on the synthesis process of Redcivir intermediate[J].Guangzhou Chemical Industry,2021,49(06):27-29.
You may like
Related articles And Qustion
See also
![1354823-36-1 N-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester](/ProductImageEN/2022-09/Small/ccca1c7e-4dcc-4cda-8d67-721d49ae5e16.png)
US $0.00-0.00/KG2025-04-04
- CAS:
- 1354823-36-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
![1354823-36-1 2-Ethylbutyl [(4-nitrophenoxy)(phenoxy)phosphoryl]-D-alaninate](/ProductImageEN/2023-09/Small/a60bffc1-7e43-4733-9697-544ce291f42b.jpg)
US $40.00/kg2025-03-07
- CAS:
- 1354823-36-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons
![1354823-36-1 N-[(S)-(4-nitrophenoxy)phenoxyphosphinyl]-L-Alanine 2-ethylbutyl ester](https://www.chemicalbook.com/CAS/20211123/GIF/1354823-36-1.gif)