Methylparaben:a preservative product
Introduction
Methylparaben is a synthetic compound used as a preservative in cosmetics, skincare, and pharmaceutical products. It prevents microbial growth, extending product shelf life. Although generally considered safe, concerns about its potential endocrine-disrupting properties have led to scrutiny. Regulatory bodies oversee its usage to ensure consumer safety. It is alkyl esters of p-hydroxybenzoic acid, and are widely used as preservatives in food, pharmaceutical and cosmetic formulation due to their low cost, high stability over a wide pH range, and antibacterial and antifungal activity. Due to the widespread usage and large amount of consumption, parabens have been widely detected in surface water, groundwater, drinking water, sewage , air and dust. Among all parabens, methylparaben was the most frequently detected chemical in various environments, including surface waters, commonly at ng/L to hundreds of µg/L1.
Application
Methylparaben, a synthetic preservative, safeguards cosmetics and pharmaceuticals from microbial growth, prolonging shelf life. Its safety, while generally affirmed, faces scrutiny for potential endocrine-disrupting properties. Regulatory oversight ensures consumer protection in its widespread usage. Methylparaben serves as a widely used synthetic preservative in various products, including cosmetics, skincare items, and pharmaceuticals. Its primary function is to inhibit the growth of microbes, thereby extending the shelf life of these products. By preventing bacterial and fungal contamination, methylparaben contributes to the overall safety and stability of cosmetic formulations, creams, lotions, and pharmaceutical preparations. Despite its efficacy in preserving product integrity, ongoing discussions surround its safety profile, prompting continuous monitoring and regulatory considerations in its application across diverse industries.
1.Methylparaben as a preservative in the development of a multi-dose HPV-2 vaccine:The human papillomavirus (HPV) vaccine is the simplest, most economical, convenient, and effective method of preventing cervical cancer. However, the current HPV vaccine is supplied as a single-dose vial with a relatively high cost per dose, which hinders its supply to low- and middle-income countries (LMICs), where the demand for HPV vaccine is highest. The results of Methylparaben as a preservative indicated that 0.12% methylparaben is the most suitable preservative for the multi-dose HPV-2 vaccine, guaranteeing the shelf life for at least three years and meeting “B” standards for antimicrobial effectiveness. The formula developed in this study can contribute toward combating cervical cancer in LMICs1.
2.The curious case of methylparaben: Anthropogenic contaminant or natural origin:The widespread use of methylparaben as a preservative has caused increased exposure to natural aquatic systems in recent decades. However, current studies have suggested that exposure to this compound can result in endocrine disrupting effects, raising much concern regarding its environmental impact. Methylparaben has also been found to be part of the metabolome of some organisms, prompting the question as to whether this compound may be more natural than previously assumed. Through a combination of field studies investigating the natural presence of methylparaben across different taxa, and a 54-day microcosm experiment examining the bioaccumulation and movement of methylparaben across different life stages of aquatic insects, results offer evidence suggesting the natural origin of methylparaben in aquatic and terrestrial biota. This study improves our understanding of the role and impact this compound has on biota and challenges the current paradigm that methylparaben is exclusively a harmful anthropogenic contaminant. Findings highlight the need for further research on this topic to fully understand the origin and role of parabens in the environment which will allow for a comprehensive understanding of the extent of environmental contamination and result in a representative assessment of the environmental risk that may pose2.
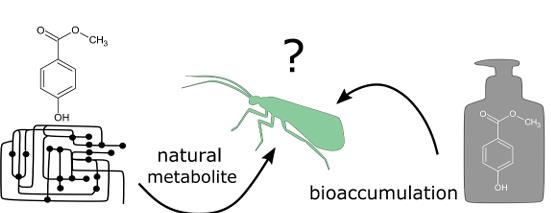
Figure 1. The curious case of methylparaben: Anthropogenic contaminant or natural origin
Synthesis
Methylparaben is typically synthesized through esterification, a chemical reaction involving the condensation of p-hydroxybenzoic acid with methanol. The process is catalyzed by acids or bases, resulting in the formation of methylparaben and water. The reaction can be represented as:

Figure 2. The synthesis of Methylparaben
This esterification reaction yields the methyl ester of p-hydroxybenzoic acid, which is the chemical structure of methylparaben. After synthesis, the compound is purified to meet the required quality standards before being utilized as a preservative in various products.
Safety
Methylparaben is generally recognized as safe for use in cosmetics and pharmaceuticals by regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the European Commission. It has a long history of use as a preservative. However, there are ongoing debates and concerns about the potential endocrine-disrupting properties of Methylparaben, including methylparaben. Some studies have suggested a weak estrogenic effect, raising questions about its impact on hormone balance. As a result, regulatory bodies continuously assess and monitor its safety. The concentrations used in products are typically low and within established limits. Individuals with specific sensitivities or concerns may choose products labeled as "paraben-free." Despite the acceptance of using parabens in food, there have been accumulating scientific evidences about their harmful effects. Overall, the safety of methylparaben is subject to ongoing scientific evaluation and regulatory oversight.
1.Methylparaben are a class of aquatic pollutants of emerging concern, among which methylparaben (MeP) causes severe pollution worldwide. However, aquatic toxicology of MeP remains largely unknown, which hinders ecological risk evaluation. In the present study, adult zebrafish were exposed to environmentally realistic concentrations (0, 1, 3, and 10 μg/L) of MeP for 28 days, with objectives to reveal the hepatotoxicity based on transcriptional, biochemical, metabolomics, and histopathological evidences. The results showed that MeP subchronic exposure induced the occurrence of hepatocellular vacuolization in zebrafish. Furthermore, degradation of biologically active molecules, including retinoic acid and estradiol, was enhanced in the liver by MeP. Overall, the present study highlights the hepatotoxicity caused by MeP pollutant even at environmentally realistic concentrations, which necessitates an urgent and accurate risk assessment3.
2. Methylparaben toxicity and its removal by microalgae Chlorella vulgaris and Phaeodactylum tricornutum:The widespread occurrence of methylparaben has aroused great concern due to its weak estrogenic endocrine-disrupting property and potential toxic effects. However, the degradation potential and pathway of Methylparaben by microalgae have rarely been reported. Here, microalgae Chlorella vulgaris and Phaeodactylum tricornutum were used to investigate their responses, degradation potential and mechanisms towards methylparaben. Two different degradation pathways of Methylparaben by the two microalgae were proposed. Methylparaben could be mineralized and completely detoxified by C. vulgaris. Overall, this study provides novel insights into Methylparaben degradation by microalgae and strategies for simultaneous biodegradation and detoxification of MPB in the environment4.
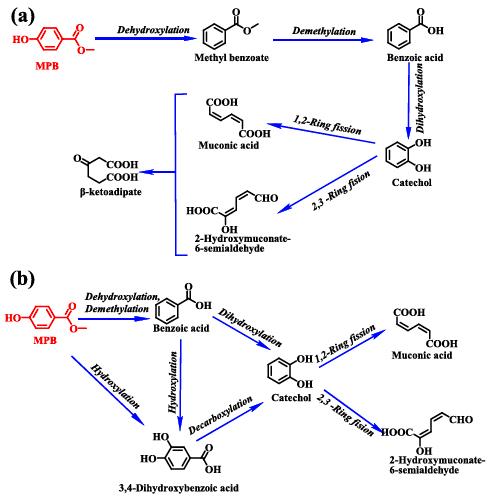
Figure 3. The proposed degradation pathway of MPB by C. vulgaris (a) and P. tricornutum (b)
Reference
1. Miao C, Ma X, Fan J, Shi L, Wei J. Methylparaben as a preservative in the development of a multi-dose HPV-2 vaccine. Hum Vaccin Immunother. 2022 Nov 30;18(5):2067421.
2. Cetini KA, GrgiI, Previi A, Roman M. The curious case of methylparaben: Anthropogenic contaminant or natural origin? Chemosphere. 2022 May;294:133781.
3. Hu C, Sun B, Tang L, Liu M, Huang Z, Zhou X, Chen L. Hepatotoxicity caused by methylparaben in adult zebrafish. Aquat Toxicol. 2022 Sep;250:106255.
4. Chang X, He Y, Song L, Ding J, Ren S, Lv M, Chen L. Methylparaben toxicity and its removal by microalgae Chlorella vulgaris and Phaeodactylum tricornutum. J Hazard Mater. 2023 Jul 15;454:131528.
You may like
Related articles And Qustion
Lastest Price from Methylparaben manufacturers

US $0.00-0.00/kg2025-04-24
- CAS:
- 99-76-3
- Min. Order:
- 1kg
- Purity:
- 98%-102%; USP
- Supply Ability:
- 1000KG

US $4.00/kg2025-04-21
- CAS:
- 99-76-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000