Correlational studies of Methylparaben
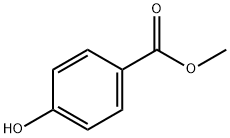
Methylparaben has been used as a preservative in the food, cosmetic, and pharmaceutical industries for over 50 years. The chemical formula for methyl paraben is C8H8O3. It is also found naturally in fruits like blueberries where it has antimicrobial activity.
Uses
Methylparaben is commonly used as a fungicide in Drosophila food media. To Drosophila, methylparaben is toxic at higher concentrations, has an estrogenic effect (mimicking estrogen in rats and having anti-androgenic activity), and slows the growth rate in the larval and pupal stages at lower concentrations.
Metabolism
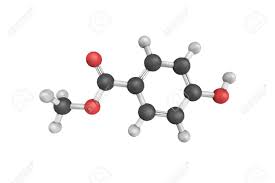
Methylparaben is completely absorbed through the skin or after ingestion, and it is hydrolyzed to para-hydroxybenzoic acid, and metabolites are rapidly excreted in the urine. There is no evidence of accumulation. It is on the FDA generally regarded as safe list.
Correlational studies
Acute toxicity studies in animals indicate that methylparaben is practically non-toxic by both oral and injectable routes. It has not been shown to be teratogenic, carcinogenic, mutagenic or embryotoxic.
It does not appear to be irritating when used topically, although some people may show cross-sensitivity if allergic to local anesthetics that are metabolized to para-aminobenzoic acid. Cases of local contact dermatitis have been reported.All parabens have similar structure to estrogen. Studies conducted in the early 2000s located traces of parabens in breast tumors, but evidence has not linked parabens with breast cancer.
The Cosmetic Ingredient Review (CIR) reviewed the safety of methylparaben, propylparaben, and butylparaben in 1984 and concluded they were safe for use in cosmetic products at levels up to 25%. Typically parabens are used at levels ranging from 0.01 to 0.3%.
References
[1] US Department of Labor. OSHA. Safety and Health Topics. Methylparaben. Accessed 8/17/2012 http://www.osha.gov/dts/chemicalsampling/data/CH_254445.html
[2] FDA’s SCOGS database; methylparaben; SCOGS-Report Number: 8; Accessed 8/17/2012. http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=scogsListing&id=210
[3] Soni MG, Taylor SL, Greenberg NA, et al. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem. Toxicol. 40:1335-73. Accessed 8/17/2012 http://www.sciencedirect.com/science/article/pii/S0278691502001072
[4] US Food and Drug Administration. Cosmetics. Parabens. Accessed 8/19/2012. http://www.fda.gov/cosmetics/productandingredientsafety/selectedcosmeticingredients/ucm128042.htm
Related articles And Qustion
Lastest Price from Methylparaben manufacturers

US $0.00-0.00/kg2025-04-24
- CAS:
- 99-76-3
- Min. Order:
- 1kg
- Purity:
- 98%-102%; USP
- Supply Ability:
- 1000KG

US $4.00/kg2025-04-21
- CAS:
- 99-76-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000