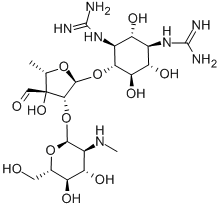
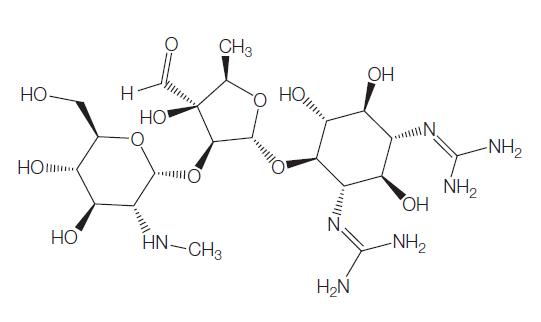
Mechanism of action of Streptomycin
Streptomycin was the first antibiotic to be effective against tuberculosis and was the first drug of any kind to be tested in a randomized controlled trial. It is a natural product produced by a soil actinomycete and was isolated from Streptomyces griseus in 1944 by Schatz et al.
Streptomycin is a member of the aminoglycoside class of antibiotics that typically consist of two or more amino-sugars covalently linked to a central aminocyclitol. However, streptomycin differs from other available aminoglycosides in that the aminocyclitol is peripherally rather than centrally located and is a streptidine, rather than the usual deoxystreptamine. Several salts of streptomycin can be produced, but streptomycin sulfate is used in human medicine. Streptomycin is a potent bactericidal antibiotic, but is also potentially nephrotoxic, vestibulotoxic, and ototoxic. The chemical structure of streptomycin is shown below.
Mechanism of action
Aminoglycosides initially associate with the cell wall and external surface of the cytoplasmic membrane and are then actively taken by an energy-dependent mechanism. The intracellular target of streptomycin has now been confirmed by X-ray crystallography to be the bacterial ribosome, where it binds to specific regions of 16S rRNA and is stabilized by the RpsL protein. Most resistance in mycobacteria in which AMEs play no role is associated with mutations in rrs and rpsl which effect streptomycin binding to its principal site of action. The binding of streptomycin to the subunit results in a misreading of mRNA codons. Consequently, faulty bacterial proteins are produced. The resultant alteration in the protein molecule may be small and it does not necessarily affect all bacterial proteins.
Therefore, this effect alone may not be lethal to bacteria, yet streptomycin and other aminoglycosides are rapidly bactericidal. Numerous hypotheses have been put forward to explain this. One likely explanation is that streptomycin causes production of abnormal membrane proteins that are essential structural components of the bacterial cell wall, leading to cell wall failure.
Bioavailability
Streptomycin, like all aminoglycosides, is a highly polar cation which strongly influences absorption, distribution, and elimination. Oral absorption is negligible, and for the treatment of systemic infections it has to be given intramuscularly or intravenously – both routes resulting in similar serum levels. Streptomycin is the only aminoglycoside to bind significantly to albumin, with approximately 34% of the drug serum protein bound.
Neurotoxicity
Streptomycin may produce flaccid paralysis directly in experimental animals. Streptomycin and other aminoglycosides can interfere with neuromuscular transmission and thereby cause postoperative respiratory depression, a drug-induced myasthenic syndrome, or unmasking or aggravation of myasthenia gravis. Aminoglycosides cause a combined presynaptic and postsynaptic block that results from interference with calcium ion fluxes at the nerve terminal (Levanen and Nordman, 1975; Argov and Mastaglia, 1979). In the past, large amounts of streptomycin (up to 5 g in adults) were sometimes introduced into the peritoneal cavity during surgery and could be absorbed from the inflamed peritoneum, causing neuromuscular blockade (McQuillen et al., 1968), which may respond to administration of calcium (Levanen and Nordman, 1975). Some patients develop circumoral paresthesiae and others rarely develop a temporary lack of mental concentration after streptomycin injections.
Lastest Price from Streptomycin manufacturers

US $0.00/kg2025-04-22
- CAS:
- 57-92-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 57-92-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt