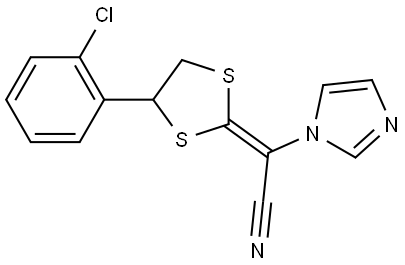
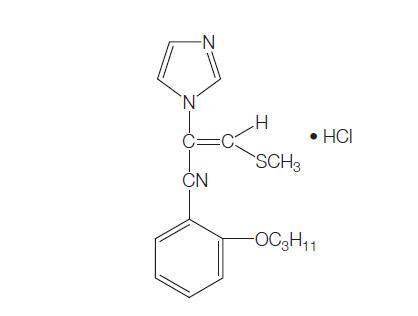
Mechanism of action of Lanoconazole
Lanoconazole is a racemic imidazole antimycotic which chemically has a dithiolan ring in addition to the imidazole ring.
It has been developed as a topical formulation and is marketed in Japan, where it is indicated for the treatment of tinea pedis, tinea corporis, and cutaneous candidiasis.

Mechanism of action
The primary mechanism of action of lanoconazole is similar to that of other imidazoles, that is, inhibition of cytochrome P450 dependent C-14a-demethylation of lanosterol, preventing conversion to ergosterol. Lanoconazole also increases collagen content and angiogenesis in newly formed granulation tissue. It has been shown to accelerate wound healing.
Pharmacokinetics and Pharmacodynamics
In dermal application studies of 14C-labeled-lanoconazole cream, applied to a shaved portion of the rats’ back, 83% of the total radioactivity was recoverable from the stratum corneum 24 hours after application. The radioactivity remained recoverable for up to 96 hours, which is approximately seven times longer than for clotrimazole and bifonazole.After 24 and 48 hours, more than 94% of the extractable radioactivity was intact drug and not a metabolite.
In humans, penetration and accumulation of lanoconazole in the horny layer are higher than those of other drugs. According to a pharmacokinetic study on lanoconazole in the horny layer of the soles in patients with tinea pedis, the concentrations were 5897315 mg/g and 4777295 mg/g after 12 and 24 hours of the application, respectively, which corresponds with 20,000 times the MIC.
Uses
Topical administration of lanoconazole in patients with common tinea pedis (interdigital type and vesicular type) is effective, and Takahashi et al. (1993) have reported beneficial effects of topical lanoconazole therapy for patients with hyperkeratotic-type tinea pedis. Tanuma et al. (2001) also investigated the activity of lanoconazole in the treatment of hyperkeratotic-type tinea pedis and compared topical administration of 1% lanoconazole cream alone (group I) with 1% lanoconazole cream and 10% urea ointment (group II). The clinical improvement rate was 70.0% (14/20) in group I and 95.7% (22/23) in group II. After 12 weeks of treatment, the fungal eradication rate was 70.0% and 95.7% in groups I and II, respectively.
Toxicity
Lanoconazole is generally well tolerated in the various clinical trials; however, several cases of contact dermatitis have been reported. Although Taniguchi and Kono (1998) reported no cross-reactivity with other imidazoles, subsequently Umebayashi and Ito (2001) reported contact dermatitis due to both lanoconazole and neticonazole ointments.
See also
Lastest Price from Lanoconazole manufacturers
US $1.10/g2021-07-17
- CAS:
- 101530-10-3
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min