Protective effect of 2-(trimethylsilyl)ethoxymethyl chloride
Background
2-(Trimethylsilyl)ethoxymethyl ether (SEM) has excellent chemical and physical properties[1]. In addition to alkali and except the most severe acidic conditions, the performance of 2-(trimethylsilyl)ethoxymethyl ether (SEM) is stable under other conditions, so it has an important role in chemical production and is very important. good research value.
Protective effect of 2-(trimethylsilyl)ethoxymethyl chloride during RNA synthesis.
In the synthesis of RNA it is critical that the 2-hydroxyl protecting group be stable throughout the various steps of the synthesis and base deprotection. At the same time, this group should also be readily removed when desired. To that end, the t-butyldimethylsilyl group has been efficacious. However, long exposure times to TBAF are required to fully remove this blocking group from the 2-hydroxyl. In addition, the bulky alkylsilyl group sterically hinders coupling, thereby requiring longer couping times. Finally, it has been shown that the TBDMS group is base labile and is partially deprotected during treatment with ethanolic ammonia.
Thus, a new 2-hydroxyl protecting group would be useful. The 2-(trimethylsilyl)ethoxymethyl ether (SEM) has appeal a suitable substitute. This protecting group is stable to base and all but the harshest acidic conditions. Therefore it is stable under all the conditions required for oligonucleotide synthesis. The SEM group can be readily introduced and the oxygen carbon bond, rather than an oxygen silicon bond, renders it unable to migrate. Finally, this protecting group can be readily removed with BF3-Etz.
The SEM group was introduced using a modification of the Moffat procedures06 (Figure 1). Treatment of S-O-dimethoxytrityl uridine (2) with dibutyltin oxide in the presence of TBAF gave the 2,3-O-dibutylstannylene derivative. Addition of SEM-Cl provided a -1:1 mixture of the 2- 4 and 3- 5 protected derivatives in 28% and 25% yield respectively. The two isomers were separated and the 2-protected nucleoside was phosphitylated to give the amidite 1 in 73% yield. The 3-protected nucleoside was succinylateci to give 6 and coupled to a polystyrene support with DCC to afford. Using standard coupling conditions, a uridine lo-mer, (Up)9U, was synthesized with an average stepwise yield of 99%.
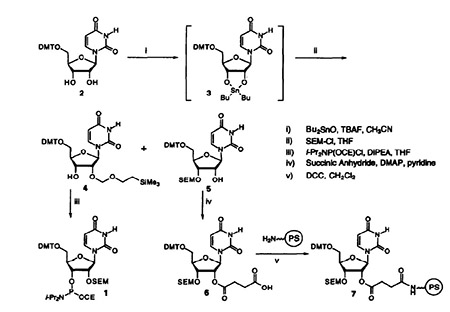
Picture 1 RNA synthesis[1]
Regioselective protection of 2-(trimethylsilyl)ethoxymethyl chloride
A remaining problem in the current synthesis of oligoribonucleosides is the slow rate of condensation due to the bulkiness of T-hydroxyl protecting groups such as t-butyldimethylsilyl (TBDMS). Another drawback is difficulty in selective protection of the T-hydroxyl of ribonucleosides with TBDMS-CI. In order to overcome these problems, we originally reported 2-(trimethylsilyl)ethoxymethyl (SEM) as a new 2-hydroxyl protecting group which could be removed by treatment with fluoride ion. Quite recently, Usman et al. has described the synthesis of (Up)9U using the SEM group where BF3.Et20 was successfully employed for removal of the SEM group. According to their method, introduction of the SEM group into the T-position of uridine was carded out via the 2',3'-O-dibutylstannylene intermediate by a modification of the procedure reported by Moffatt. However, this method gave a nearly 1:1 mixture of the 2'- and 3'-isomers. In this paper, we report a highly efficient and general procedure for the synthesis of 2'-O-SEM protected ribonucleoside phosphoramidite building blocks.
It was reported that the di(t-butyl)silanediyl group can be rapidly deprotected under very mild conditions by use of pyridinium poly(hydrogen fluoride) or Bu3NHF. On the other hand, we found that the SEM group of 2'-O-SEM-protected ribonucleosides were sufficiently stable to these desilylating reagents. Therefore, we used 3',5'-O-di(t-butyl)silanediylnucleosides (picture 2a-e) as starting materials for the 2'-O-SEM protection. These key intermediates could be obtained in 84-99% yields from the parent nucleosides la-e.
When compound 2b was allowed to react with 2-(trimethylsilyl) ethoxymethyl chloride (SEM-CI) in the presence of i-Pr2NEt and Bu4N112 in CH2Cl2 for 3 h, the corresponding 2'-O-SEM protected derivative 3b was obtained in 88% yield. When the N3-unprotected uridine derivative 2a was used in place of 2b, the SEM group was introduced exclusively at the N3-position. In the case of the 6-N-benzoyladenosine derivative 2e, the SEM group was introduced to the T-hydroxyl, but the 6-N or N 1 position was simultaneously alkylated to a degree of 30-40%. Predominant base-modification with SEM-CI was also observed in the case of 4-N[1]benzoylcytidine and 2-N-phenylacetylguanosine derivatives (picture 2).
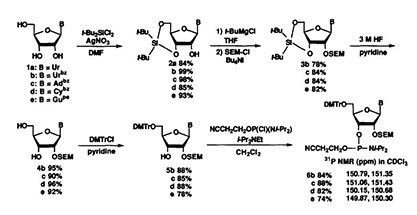
Picture 2 Regioselective protection[1]
Reference
1 Wincott F E, Usman N. 2′-(Trimethylsilyl) ethoxymethyl protection of the 2′-hydroxyl group in oligoribonucleotide synthesis[J]. Tetrahedron letters, 1994, 35(37): 6827-6830.
See also
Lastest Price from 2-(Trimethylsilyl)ethoxymethyl chloride manufacturers

US $0.00-0.00/KG2025-07-07
- CAS:
- 76513-69-4
- Min. Order:
- 1KG
- Purity:
- 95.0%
- Supply Ability:
- 10000KGS

US $10.00/KG2025-04-21
- CAS:
- 76513-69-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt