Mechanism of action of Isoniazid
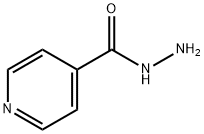
Isoniazid, isonicotinic acid hydrazide (INAH or INH), was discovered independently in 1952 at both Squibb and Roche Laboratories. Animal studies showed that isoniazid was a very potent antituberculosis drug, and subsequent clinical trials confirmed its high efficacy for the treatment of human tuberculosis. Isoniazid inhibits mycolic acid synthesis and has potent early bactericidal activity against Mycobacterium tuberculosis. It is relatively nontoxic, inexpensive, and remains an important first-line drug for the treatment of tuberculosis.

Mechanism of action
Isoniazid inhibits the synthesis of mycolic acids, a critical component of the lipid-rich mycobacterial cell wall, and has a bactericidal action on M. tuberculosis. Isoniazid is a prodrug that is converted by the mycobacterial enzyme catalase peroxidase katG into the active form. Various radicals of isoniazid then covalently bind to nicotinamide adenine dinucleotide (NAD), and this inhibits the product of the inhA gene, an NADHdependent enoyl-acyl carrier protein reductase that is part of the fatty acid synthase (FAS)-II responsible for mycolic acid synthesis.
Resistance to isoniazid can therefore arise by mutations in either katG or inhA and, because inhA also appears to be the target of ethionamide, cross-resistance can occur between these two drugs. In one study that included 403 isoniazid-resistant M. tuberculosis isolates obtained from six different countries, 46% of strains had a mutation in codon 315 of katG (katG315), and in a further 12%, mutations associated with the inhA gene, particularly in the promoter region, were found. Mutations in katG typically correlate with high-level resistance and inhA mutations with low-level resistance.
Multiple mutations in other genes [alkyl hydroperoxide reductase (ahpC), NADH dehydrogenase (ndh), and ketoacyl synthase (kasA)] have been found in isoniazid-resistant M. tuberculosis isolates; however, mutations in these genes also occur in isoniazid-sensitive M. tuberculosis isolates, and only ahpC promoter mutations correlated well with isoniazid resistance As multiple mutations are associated with isoniazid resistance, rapid genotypic resistance testing is more difficult than for rifampicin. However, a multiplex polymerase chain reaction (PCR)–reverse hybridization assay that includes probes to the katG315 and inhA promoter regions was approximately 90% sensitive in detecting isoniazid resistance.
Bioavailability
Isoniazid is rapidly and nearly completely absorbed from the gastrointestinal tract, and the peak serum level is reached 1–2 hours after administration. After a single dose of 12.5 mg/kg body weight (800–1000 mg in an adult), the peak level is 10–15 mg/ml. A similar peak serum level is attained by all patients at this time, but 4–6 hours after administration serum levels differ according to whether the subject is a rapid or slow isoniazid inactivator. In the former, serum levels at 6 hours approach zero, whereas in the latter a level of 5 mg/ml is still present. The serum half-life of isoniazid in slow inactivators is 2–4 hours and in rapid inactivators 0.5–1.5 hours.
Toxicity
Hepatotoxicity Originally it was considered that liver damage due to isoniazid was rare, mild, and transient. It is now apparent that isoniazid frequently causes asymptomatic transaminitis and that it is an occasional cause of severe clinical hepatitis. Prior to the widespread use of isoniazid for preventive therapy, hepatotoxicity was probably attributed to other antituberculosis drugs, all of which, with the possible exception of streptomycin, can cause hepatitis. The risk of developing clinical hepatitis, particularly fatal hepatitis, during what was once regarded as standard chemotherapy of tuberculosis based on isoniazid was very small.
In clinical trials performed in the period 1953–1973, which used isoniazid combined with streptomycin, PAS, and thiacetazone, the incidence of hepatitis was less than 2%, but routine liver function tests were not performed in most of these trials. Where drug-induced hepatitis did occur, PAS or thiacetazone seemed more likely causes than isoniazid because the incidence of hepatitis was higher when PAS and thiacetazone were used than when isoniazid was given alone or with streptomycin.
Lastest Price from Isoniazid manufacturers

US $0.00/KG2025-04-24
- CAS:
- 54-85-3
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG

US $0.00/Kg/Drum2025-04-21
- CAS:
- 54-85-3
- Min. Order:
- 1KG
- Purity:
- 99%-101%; EP/BP
- Supply Ability:
- 1000KGS