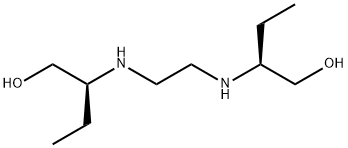
Side-effects of Ethambutol
Ethambutol was discovered at Lederle Laboratories in 1961, when randomly selected synthetic compounds were being tested for antituberculosis activity. Chemically, it is dextro-2, 2u-(ethylenediimino)- di-1-butanol dihydrochloride, and it has a high degree of activity against M. tuberculosis.Ethambutol is a useful drug for the treatment of tuberculosis and infections due to Mycobacterium avium complex and other nontuberculous mycobacteria.

Uses
Ethambutol is now a first-line drug for treating all forms of tuberculosis. Ethambutol is included in initial treatment regimens, primarily to prevent emergence of rifampicin resistance when primary resistance to isoniazid may be present. The recommended initial treatment regimen for all adults with previously untreated tuberculosis is isoniazid, rifampicin, pyrazinamide, and ethambutol. If drug susceptibility test results are known and the M. tuberculosis isolate is susceptible to isoniazid and rifampicin, ethambutol may be omitted. Ethambutol can be ceased as soon as drug susceptibility test results have demonstrated that the isolate is susceptible to the other first-line agents.
Excretion
Most of absorbed ethambutol (about 80%) is excreted via the kidneys as the active unchanged drug. This excretion occurs within 24 hours of administration and high concentrations of the active drug are attained in urine. Some 8–15% of absorbed ethambutol is converted to various inactive metabolites and these are also excreted in the urine. The rate of ethambutol metabolism is similar in all individuals and it is not altered after prolonged administration. Unabsorbed ethambutol, about 20% of an oral dose, is excreted unchanged in the feces.
Side-effects
Allergic reactions with ethambutol are rare. Hypersensitivity, manifested by fever, rash, hypotension, and liver dysfunction, attributable to ethambutol, has been reported. Reports of skin reactions include erythema multiforme-type eruption with eosinophilia and liver dysfunction, lichenoid eruption, and toxic epidermal necrolysis. There are two reports of pneumonitis and eosinophilia after commencement of ethambutol, isoniazid, and rifampicin, which recovered after ethambutol was ceased. There are rare reports of hematologic abnormalities associated with ethambutol, including thrombocytopenia, neutropenia, and neutropenia with eosinophilia.
Elevated serum uric acid levels occur in about two-thirds of patients treated with ethambutol; generalized arthralgia and acute gout has been observed in association with hyperuricemia. False-positive phentolamine tests for phaechromocytoma have been observed in patients receiving ethambutol, presumably because of some interaction between these substances. Ethambutol is not considered hepatotoxic, although there is one case of jaundice and cholestasis. Indeed, ethambutol is recommended for use in patients who develop hepatotoxicity after commencement of antituberculous therapy.
You may like
Lastest Price from Ethambutol manufacturers

US $0.00/KG2025-04-24
- CAS:
- 74-55-5
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1000KG

US $999.00-666.00/ton2025-04-21
- CAS:
- 74-55-5
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000ton