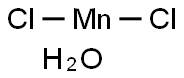Manganese Chloride Tetrahydrate: A Versatile Compound in Chemistry
Introduction
Manganese chloride tetrahydrate (MnCl₂·4H₂O) is a widely recognized and essential compound in the field of chemistry, known for its versatility, stability, and broad range of applications. As a manganese salt with four molecules of water, this compound appears as pink crystalline solids, commonly used across industrial, laboratory, and research contexts.

Figure 1 Characteristics of Manganese chloride tetrahydrate
Properties of Manganese Chloride Tetrahydrate
Manganese chloride tetrahydrate exhibits distinct physical and chemical properties that make it a valuable compound in chemistry. Physically, it is a pink crystalline solid with a melting point of around 58°C. When heated, it decomposes to form manganese(II) chloride anhydrous, releasing water molecules in the process. The compound is soluble in water, producing a pale pink solution, which makes it particularly useful in aqueous reactions.
Chemically, manganese chloride tetrahydrate functions as a source of Mn²⁺ ions, which are essential in various redox reactions. The compound demonstrates paramagnetic properties due to the unpaired electrons in the manganese ion, making it useful in certain magnetic resonance applications. Additionally, manganese chloride tetrahydrate can act as a Lewis acid, enabling it to catalyze a range of organic reactions, including oxidation and coupling reactions.
Manganese chloride tetrahydrate's chemical reactivity also extends to coordination chemistry, where it forms complex compounds with ligands such as amines, phosphines, and carboxylates. This reactivity is harnessed in various synthesis processes, particularly in the preparation of manganese-based catalysts and materials. The compound's versatility in chemical reactions underscores its importance in both industrial and academic research settings.
Composition and Main Components
The molecular composition of manganese chloride tetrahydrate can be expressed by the chemical formula MnCl₂·4H₂O. The compound consists of one manganese atom (Mn), two chlorine atoms (Cl), and four water molecules (H₂O) coordinated with the manganese ion. The presence of water molecules in the crystal structure distinguishes the tetrahydrate form from the anhydrous manganese chloride, giving it unique properties and reactivity.
The manganese ion in manganese chloride tetrahydrate exists in the +2 oxidation state (Mn²⁺), which is the most stable oxidation state for manganese in this compound. The Mn²⁺ ion is octahedrally coordinated by two chloride ions and four water molecules, forming a stable structure that contributes to the compound's solubility and reactivity in water.
In terms of purity, commercially available manganese chloride tetrahydrate typically contains small amounts of impurities, including trace metals and other anions. However, high-purity grades of the compound are available for use in sensitive applications, such as analytical chemistry and advanced material synthesis. The choice of grade depends on the specific requirements of the application, with higher purity levels being preferred for precision work.
Applications of Manganese Chloride Tetrahydrate
Manganese chloride tetrahydrate finds application across a wide range of fields, from industrial manufacturing to research and development. Its ability to serve as a source of Mn²⁺ ions and its solubility in water makes it particularly valuable in several chemical processes.
1. Industrial Uses:
In the industrial sector, manganese chloride tetrahydrate is utilized in the production of dry cell batteries, where it serves as an essential component of the cathode material. The compound also plays a role in the synthesis of manganese-based pigments and dyes, which are used in ceramics, glass, and textiles. Additionally, manganese chloride tetrahydrate is used in the preparation of other manganese compounds, such as manganese(III) oxide (Mn₂O₃) and manganese dioxide (MnO₂), which are important in various industrial applications.
2. Water Treatment:
Another important application of manganese chloride tetrahydrate is in water treatment processes. The compound is used as a precursor to manganese-based coagulants and oxidizing agents, which help remove impurities and contaminants from water. Manganese compounds are particularly effective in removing iron and sulfur from water supplies, making them valuable in both municipal and industrial water treatment facilities.
3. Catalysis:
In the field of catalysis, manganese chloride tetrahydrate is employed as a catalyst or a catalyst precursor in a variety of chemical reactions. It is commonly used in organic synthesis, where it catalyzes reactions such as oxidation, coupling, and cyclization. Manganese-based catalysts are often chosen for their ability to promote environmentally friendly and sustainable chemical processes, making manganese chloride tetrahydrate a valuable compound in green chemistry.
4. Research and Development:
Manganese chloride tetrahydrate is widely used in academic and industrial research settings due to its versatility in chemical reactions. It is a common reagent in coordination chemistry, where it is used to synthesize a variety of metal-organic frameworks (MOFs) and coordination complexes. These materials have potential applications in gas storage, catalysis, and drug delivery, making manganese chloride tetrahydrate an important compound in materials science research.
5. Biological Applications:
In biochemistry and molecular biology, manganese chloride tetrahydrate is used as a source of manganese ions, which are essential cofactors for various enzymes. Manganese ions play a critical role in the catalytic activity of enzymes involved in processes such as photosynthesis, respiration, and DNA synthesis. As a result, manganese chloride tetrahydrate is often used in studies of enzyme function and activity, as well as in the production of certain biomolecules.
Storage Guidelines for Manganese Chloride Tetrahydrate
Proper storage of manganese chloride tetrahydrate is essential to maintain its stability and prevent degradation. The compound should be stored in a cool, dry place, away from moisture and direct sunlight. Exposure to air and humidity can lead to the gradual loss of water molecules from the crystal structure, resulting in the formation of manganese chloride anhydrous, which has different properties and reactivity.
It is important to store manganese chloride tetrahydrate in tightly sealed containers to prevent moisture absorption and contamination. The use of desiccants in the storage area can help control humidity levels and protect the compound from hydration changes. In laboratory settings, manganese chloride tetrahydrate should be stored in chemical storage cabinets that are designed to minimize exposure to air and moisture.
Additionally, safety precautions should be taken when handling and storing manganese chloride tetrahydrate. The compound can be harmful if ingested, inhaled, or absorbed through the skin, and it may irritate the eyes, skin, and respiratory system. Proper personal protective equipment (PPE), such as gloves, goggles, and lab coats, should be worn when handling the compound, and it should be used in well-ventilated areas to minimize exposure to dust and fumes.
References:
[1] TORKEL B BRISMAR. Oral Manganese Chloride Tetrahydrate: A Novel Magnetic Resonance Liver Imaging Agent for Patients With Renal Impairment: Efficacy, Safety, and Clinical Implication.[J]. Investigative Radiology, 2024. DOI:10.1097/RLI.0000000000001042.[2] NILS ALBIIN. Manganese chloride tetrahydrate (CMC-001) enhanced liver MRI: evaluation of efficacy and safety in healthy volunteers.[J]. ACS Applied Bio Materials, 2012. DOI:10.1007/s10334-012-0307-x.
See also
Lastest Price from Manganese chloride tetrahydrate manufacturers

US $0.00/kg2025-09-29
- CAS:
- 13446-34-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00/KG2025-04-21
- CAS:
- 13446-34-9
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month