Malachite synthesis
Malachite, a mineral of copper, is one of the most beautiful minerals known. In the natural state, this relatively soft mineral usually shows various tints of green, varying from a dark, rich green to bright Kelly green. Since earliest civilizations, it has been carved into art forms or semiprecious jewelry, or ground into a fine powder for use as an artist's pigment.

Chemically, Malachite is a combination of copper(II) carbonate and copper(II) hydroxide, CuCO3xCu(OH)2 .
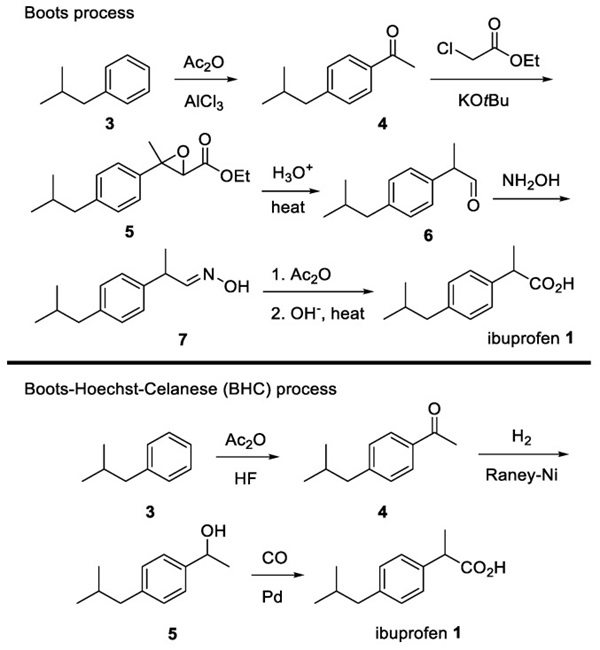
Malachite is synthesized using the following reaction:
2CuSO4·5H2O(aq) + 2Na2CO3(aq) à CuCO3Cu(OH)2(s) + 2NA2SO4(aq) + CO2 + 9H2O
The Malachite mixture was made by adding 3.10g of CuSO4.5H2O to 12.5 mL of distilled water, which was then stirred using a stir plate. A second solution of 1.45g of Na2CO3 and 25mL of Distilled water was then added to the original mixture slowly with constant stirring. The new mixture was then placed, while still in the beaker, into a 400 mL beaker filled with an ice bath for 30 minutes.
The solution was then poured into a funnel lined with filter paper placed in a flask and sat for 20 minutes. Distilled water was used to ensure that no solid was sticking to the sides of the beaker or funnel. The precipitate was then left in the top of the funnel with the liquid in the waste flask.
You may like
See also
Lastest Price from Copper(II) sulfate pentahydrate manufacturers

US $0.00-0.00/kg2025-11-21
- CAS:
- 7758-99-8
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 1000kg
US $0.00/kg2025-11-13
- CAS:
- 7758-99-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS