Main Differences between 1 Butanol and 2 Butanol
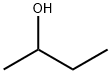
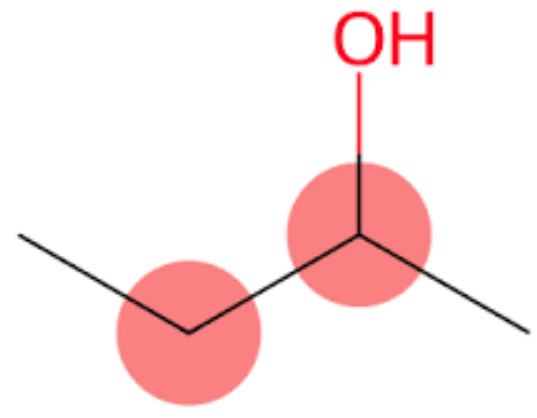
2-Butanol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3.
2-Butanol is widely used as a solvent for domestic cleaning agents and paint removers. It is also utilized in perfumes and artificial flavors. It plays an important role in the extraction of fish meal to produce fish protein concentrate. It is involved in the production of fruit essences. It plays an essential role in the esterification of acetic acid. It is useful for the derivatization of chlorinated hydroxyfuranones. It is also used as a precursor for methyl ethyl ketone.
The type of intermolecular force present in 2-butanol is hydrogen bonding which occurs due to the presence of an electronegative atom like Oxygen (O) directly attached to a proton.
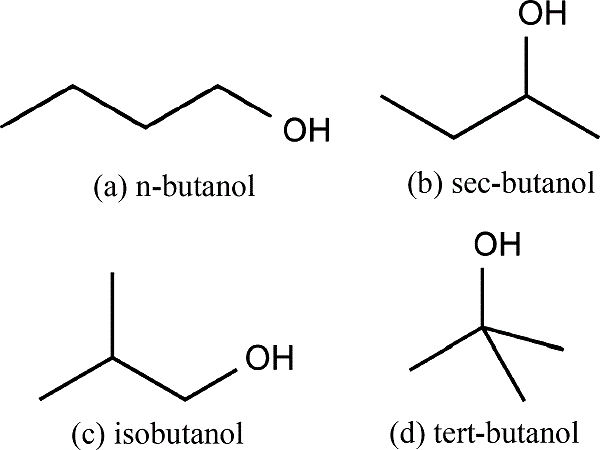
Butanol is an alcohol. It is an organic compound having the functional group –OH attached to a carbon atom. Butanol has four carbon atoms. The general formula of butanol is C4H9OH. This formula has five isomeric structures. Isomers are molecules with the same molecular formula but different chemical structures. 1 Butanol and 2 butanol are two of these isomers. The main difference between 1 Butanol and 2 Butanol is that 1 butanol has the –OH group attached to the terminal carbon of the molecule whereas 2 butanol has the –OH group attached to the second carbon atom.
Related articles And Qustion
See also
Lastest Price from 2-Butanol manufacturers

US $0.00/KG2025-04-21
- CAS:
- 78-92-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $5.00-2.00/KG2024-10-11
- CAS:
- 78-92-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg