Is 2-butanol the same with N-butanol?
No, they are different. They are butanol isomers, and the location of the hydroxyl group is different.
Fermentation by-product congeners are primarily a result of the fermentation and aging process, or they are added during production. The quantities of each alcohol congener depend on the amount and type of beverage consumed. The most commonly encountered alcohol congeners are listed in order of importance as follows: methanol, isopropanol, 1-propanol, 1-butanol, 2-butanol, isobutanol, 2-methyl-1-butanol and 3-methyl-1-butanol.
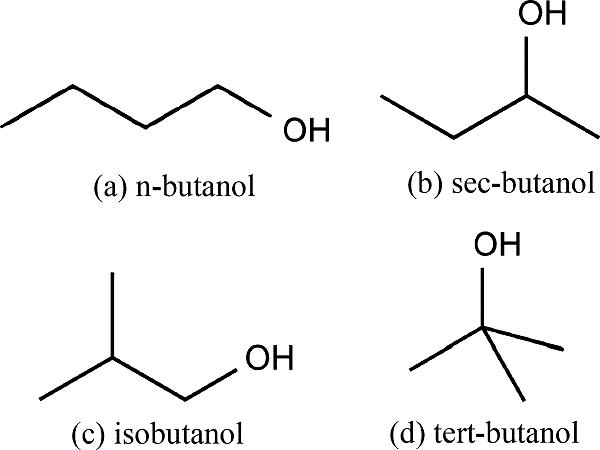
There are 4 different butanol isomers, including 1-butanol (n-butanol), 2-butanol, iso-butanol and ter-butanol, depending on the location of the hydroxyl group (–OH) and carbon chain structure. n-Butanol has a straight-chain structure with the alcohol at the terminal carbon. Sec-butanol is also a straight-chain molecule but the OH group is attached to an internal carbon. Isobutanol is a branched isomer with the OH group at the terminal carbon, while tert-butanol refers to the branched isomer with the OH group at an internal carbon.
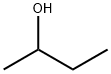
2-butanol
IUPAC name: Butan-2-ol
Other names: sec-Butanol, sec-Butyl alcohol, 2-Butanol, 2-Butyl alcohol.
n-butanol.
IUPAC name: Butan-1-ol
Other names: 1-Butanol, n-Butyl alcohol, n-Butyl hydroxide, n-Propylcarbinol.
You may like
Related articles And Qustion
See also
Lastest Price from 2-Butanol manufacturers

US $0.00/KG2025-04-21
- CAS:
- 78-92-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $5.00-2.00/KG2024-10-11
- CAS:
- 78-92-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg