Magnesium Carbonate:applications in industrial and medical fields
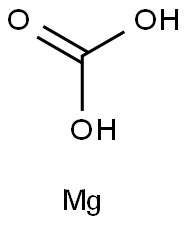
Magnesium carbonate is an inorganic compound with the molecular formula MgCO3. Its molecular weight was 84.31, the relative density was 3.037, and the appearance was white granular powder. It decomposes at 350°C and into carbon dioxide at 700°C. Magnesium carbonate is slightly soluble in cold water and acid. Its trihydrate is a colorless needle-like crystal with a melting point of 165℃ and a relative density of 1.850. Its pentahydrate is a white monoclinal crystal with a relative density of 1.73, which is decomposed by heat in air. It can be decomposed by heat in air. Magnesium carbonate can be prepared by adding sodium carbonate and carbon dioxide to magnesium brine solution. Its hydrate can be obtained by drying at 50 ° C and below under anhydrous conditions. Magnesium carbonate can be used as refractory material, boiler, pipeline insulation material and food, medicine, cosmetics, rubber, ink additives. Not only is magnesium carbonate widely used in industry, in the medical industry, magnesium carbonate is used as a novel gastric combined bile acid drug. Magnesium carbonate has dual effects of anti-acid and bile neutralization, and is commonly used in the clinical treatment of bile reflux disease and gastric ulcer[1].

Figure 1. Magnesium carbonate
Physical and Chemical Properties
Due to the different crystallization conditions of alkaline hydrated magnesium carbonate and adjacent hydrated magnesium carbonate, some of the crystallized products are light and some are heavy. The lighter is MgCO3-H2O. The heavier ones are :5MgCO3-Mg(OH)2 -3H2O, 5MgCO3-2Mg(OH)2 -7H2O, 4MgCO3-Mg(OH)2, and 3Mg(OH)2 -4H2O. Magnesium carbonate is a trihydrate salt at room temperature. Light magnesium carbonate is a loose white powder with no flavor. It has a relative density of 2.2 and a chemical melting point of 350 ° C. "Magnesium carbonate is stable in air, but when heated to 700 ° C, it breaks down to produce carbon dioxide, producing magnesium oxide, which is almost insoluble in water but produces a slightly alkaline reaction in water." Magnesium carbonate is insoluble in ethanol but can be dissolved by dilute acid and bubbling. Light magnesium carbonate is mainly used as filler and reinforcing agent for transparent or light colored rubber products. Magnesium carbonate mixed with rubber almost does not change the refractive index of rubber itself, and can improve the wear resistance, bending resistance and tensile strength of rubber. It is mainly used as an additive in paints, inks and coatings, and is also widely used in the toothpaste, pharmaceutical and cosmetic industries[2].
Uses
1, Magnesium carbonate is used in the manufacture of magnesium salt, magnesium oxide, fireproof paint, ink, glass, toothpaste, rubber filler, etc. It is used as flour improver and bread puffing agent in food [3].
2, Magnesium carbonate is used in the treatment of bile reflux disease and gastric ulcer.
3, Magnesium carbonate used as alkaline agent, desiccant, color protectant, anti-caking agent, carrier, expansion agent, acidity regulator. EEC certified magnesium carbonate can be used in salt, sugar powder, acidified thin cream, milk, ice cream, biscuits. Magnesium carbonate residues in food must not exceed 0.5%(Japan). It can also be used as a chemical filler [4].
Pharmacological effects and mechanism of action
1, Bile reflux disease
Magnesium carbonate is a novel bile acid-binding drug with a unique layered reticular structure. Its active component, magnesium carbonate, is released after decomposition in the stomach and has dual effects of acid resistance and bile neutralization. Therefore, magnesium carbonate is a commonly used drug in the clinical treatment of bile reflux diseases, with few side effects and high safety. In vitro experiments confirmed that magnesium carbonate could selectively and reversibly bind bile acids under acidic conditions. The ability of magnesium carbonate to adsorb bile acids is related to the polarity of the bile acid components over a wide pH range. The adsorption effect of magnesium carbonate on DCA, CDCA and other less polar bile acid fractions was stronger. At pH 7, magnesium carbonate still has a certain adsorption rate for bile acids. The pH of the terminal ileum was 7.49, and the pH of the posterior cecum dropped sharply to 6.4. The pH gradually increased from the right to the left half of the colon, eventually reaching a mean value of 7.0. The pH of the rectum is probably between 8 and 10. Magnesium carbonate can bind to colonic bile acids, which is beneficial to reduce the exposure of colonic bile acids and improve intestinal microecology. Magnesium carbonate can alleviate the symptoms of patients with bile acid-related diarrhea to a certain extent and improve the quality of life of patients[5].
2, Treatment of gastric ulcer
In recent years, magnesium carbonate has been widely used as a protective agent for gastric mucosa. Its mechanism of action is as follows :(1) Magnesium carbonate can quickly neutralize gastric acid, ensure the optimal pH value in the stomach, and maintain the physiological environment of the stomach. (2) Magnesium carbonate promotes the secretion of bicarbonate and enhances the function of gastric mucosal barrier. (3) Magnesium carbonate combines with bile acids in the stomach in an acidic environment. Bile acids bound to magnesium carbonate are released after entering the alkaline environment of the gut and do not affect the intestinal and hepatic circulation of bile acids. (4) Magnesium carbonate adsorbed and neutralized all the proteins and cytotoxic substances secreted by Helicobacter pylori, and inhibited its destructive effect. (5) It has a good therapeutic effect on bile reflux gastritis and gastric mucosal injury caused by non-steroidal anti-inflammatory drugs[6].
Synthesis
At present, there are three common synthesis methods of magnesium carbonate:
1, After adding water to the mud, CO2 was transferred to magnesium bicarbonate and then filtered, and the filtrate was heated to obtain carbonate base precipitation. Further purification can obtain pure magnesium carbonate[7].
2, Heat dolomite (MgCO3-CaCO3) with low fire. At 0.508-0.609 MPa, magnesium oxide becomes magnesium carbonate, which is dissolved by interaction with water and carbon dioxide[8].
3, The same amount of crystalline magnesium sulfate and crystalline sodium carbonate are dissolved in 10 times the amount of water, heated to 60 ~ 80℃ to fully mix. At this point, carbon dioxide gas is produced to produce precipitation. The precipitate was filtered off and then mixed with hot water at 70 to 80 ° C and filtered again. Repeat the washing process. After thorough washing, the precipitate was dried at 50 to 60°C.
References
[1]Lundasen T, Galman C, Angelin B, et al.Circulating intestinal fibroblast growthfactor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesisin man[J]. J Intern Med, 2006,260(6):530-536.
[2]Walters JR,Tasleem A M,Omer O s, et al. A new mechanism for bile aciddiarrhea: defective feedback inhibition of bile acid biosynthesis[J].Clin GastroenterolHepatol, 2009,7(11):1189-1194.
[3]Chey w D, Camilleri M, Chang L, et al. A randomized placebo-controlled phasellb trial of a3309,a bile acid transporter inhibitor,for chronic idiopathicconstipation[J].Am J Gastroenterol, 2011,106(10):1803-1812.
[4]Montagnani M,Love M W,Rossel P, et al. Absence of dysfunctional ilealsodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathicbile acid malabsorption[J]. Scand J Gastroenterol, 2001,36(10):1077-1080.
[5]Sadik R, Abrahamsson H, Ung K A, et al.Accelerated regional bowel transit andoverweight shown in idiopathic bile acid malabsorption[J]. Am J Gastroenterol,2004,99(4):711-718.
[6]Wong B S, Camilleri M, Carlson P J, et al.A Klothobeta variant mediates proteinstability and associates with colon transit in irritable bowel syndrome with diarrhea[J].Gastroenterology, 2011,140(7):1934-1942.
[7]Kawamata Y, Fujii R, Hosoya M, et al.A G protein-coupled receptor responsiveto bile acids[J].J Biol Chem, 2003,278(11):9435-9440.
[8]Camilleri M. Peripheral mechanisms in irritable bowel syndrome[J]. N Engl JMed,2012,367(17):1626-1635.
You may like
Related articles And Qustion
See also
Lastest Price from Magnesium carbonate manufacturers

US $3.50/kg2025-04-18
- CAS:
- 13717-00-5
- Min. Order:
- 1kg
- Purity:
- ≥98%
- Supply Ability:
- 3000tons/month

US $0.00-0.00/KG2025-04-15
- CAS:
- 13717-00-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg