Magnesite: General properties; Occurrence; Mining and Industrial applications
General properties
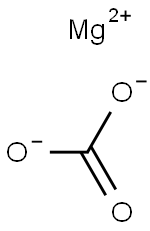
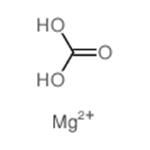
Magnesite (MgCO3) is like alumina, that is, it is considered either as an ore for magnesium metal production or as an industrial mineral. When pure, magnesite contains 47.8 wt.% magnesium oxide (MgO) and 52.2 wt.% carbon dioxide. Natural magnesite almost always contains some calcite (CaCO3) and siderite (FeCO3). Magnesium also occurs in dolomite [FeMg(CO3)2], a sedimentary rock in which MgCO3 constitutes 45.65 wt.% (i.e., 21.7 wt.% MgO) and 54.35 wt.% CaCO3.

Magnesite color varies from white, when pure, to yellowish or gray white and brown. Its Mohs hardness ranges from 3.5 to 4.5 and its density varies from 3000 to 3200 kg.m–3. A vitreous luster and very slow reaction with cold acids distinguishes magnesite from other carbonates. Magnesite, dolomite, seawater, and lake brines are used as major sources of magnesium metal with the most common source being lake brines and seawater.
Occurrence
Magnesite occurs in two physical forms: as cryptocrystalline or amorphous magnesite and as macrocrystalline magnesite. It occurs in five different ways: as a replacement mineral in carbonate rocks; as an alteration product in ultramafic rocks (e.g., serpentinite, dunite); as a vein-filling material; as a sedimentary rock; and as nodules formed in a lacustrine environment. Replacement-type magnesite deposits involve magnesium-rich hydrothermal fluids entering limestone via openings to produce both magnesite and dolomite. The alteration-type deposits are formed by the action of carbon-dioxide-rich waters on magnesium-rich serpentinite. Sedimentary deposits usually occur as thin layers of variable magnesite quality. Lacustrine magnesite deposits consist of nodules of cryptocrystalline magnesite formed in a lake environment. Both vein filling and sedimentary magnesite occurrences are rarely mined on a large scale.
Mining
All magnesite deposits are mined by open-cut methods. During mining the strip ratio, that is, the quantity of magnesite ore to waste material, may be high. The processing of magnesite ore begins with crushing, screening, and washing. The estimated world economic reserves of magnesite are about 8.60 billion tonnes expressed as MgCO3. China is ranked first, followed by Russia and North Korea.
Industrial applications
Raw magnesite is used for surface coatings, landscaping, ceramics, and as a fire retardant.
Related articles And Qustion
Lastest Price from Magnesium carbonate manufacturers

US $1200.00-1100.00/ton2025-09-23
- CAS:
- 546-93-0
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M
US $3.00-9.00/KG2025-06-19
- CAS:
- 546-93-0
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available